Summer loving is in the air, so what better time to think about sex? But we're not going to get graphic - we're talking about the genetics of sex determination. Plus, why turkeys need a wingman, figuring out fingerprints, and a leggy gene of the month.
In this episode

00:56 - Professor Judith Mank - Sex evolution
Professor Judith Mank - Sex evolution
with Professor Judith Mank - UCL
Kat - Genetically speaking, our human species is broadly divided into male and female, determined by our sex chromosomes - XX for females and XY for men, although there are people whose chromosomes don't strictly conform to this, for various reasons. But the way that we do sex - at least on a genetic level - isn't the only way to do it. To find out more, I spoke to Judith Mank, Professor of Evolutionary and Comparative biology at University College London. I started by asking her what, biologically speaking, do we actually mean by "sex"?
Judith - So, sex can be two things. So, it's the act of actual - it's called syngamy, the combination of egg and sperm, but related to that, what we said is the evolution of sexual dimorphism so how you get separate males and females. Males and females often look different, they act different, and that's why we study it in group.
Kat - A lot of us are familiar with the idea that humans have an X and a Y chromosome if you're a man, and XX, if you're a female. Is this the only way of doing it?
Judith - No, there's a million different ways. There are a lot of fish that actually flip partly through their life. So, they start out as one sex and when there's some sort of cue, they flip to the other one. Interestingly, it seems that within a couple of hours of the cue, their brain starts changing. They start acting like the other sex and it takes about 3 days after that for the gonad to follow.
Kat - Their body completely changes as well to the other gender?
Judith - Yep, and they start reproducing within about 3 days with the other sex. There are a lot of animals that are XY like mammals are, but different sort of independent X and Y chromosomes. We study in our group, we do a lot of work on birds which are Z and W. So, in that case, the male has two Z chromosomes and the female has one Z and one W. So, the W is quite somewhere to the Y that you see in humans.
Kat - Now, this seems quite strange that there would be so many different ways of doing this in the natural kingdom. How has this arisen?
Judith - That's actually the big question at the moment to be honest. So, no one really understands how some things are fundamental as how you determine sex changes. The only thing that we do understand is this old idea of conservation - so, you've got the same sex determining system in most mammals. You've got the same system in birds. You've got the same system in fruit flies. That idea that sex should be conserved is clearly not true. So, you've got some systems like fish where within populations, you've got multiple mechanisms of sex determination and it changes very, very quickly. That seems to be much more common.
Kat - If you're going to have sexual reproduction, you need a male and female or two different things to make it work, producing eggs and sperm. Are there any inklings of maybe how these kind of mechanisms of the sex chromosomes have arisen at least in some of the animals or some of the creatures you studied?
Judith - So, there's a lot of theory. Not a lot of it is really well proved, but most people think that if you've got a gene that determines sex, say, a gene that makes you male. And there could be several different genes that might predispose you to being male, but if that gene is linked - is physically very close to another gene that confers some male benefit - so, if you have both of them, you're both a male and you're a very fit male, you're a good male then that sort of sends you along the path to having a Y chromosome. But if you have a gene that makes you female and that's close to another gene that makes you a very good female then that would sort of send you on the path to a W chromosome.
Kat - What is the advantage of actually having two sexes because you think about well, something like an amoeba where it just splits itself, it reproduces, seems to be kind of fine, bacteria seem kind fine? What is the actual advantages of having different sexes?
Judith - Well, there's two things. So one, some things have sex but don't have obligate sex. They don't always have to have sex. They can sometimes reproduce clonally.
Kat - Like a yeast?
Judith - Like a yeast, some fish, a lot of plants. So, occasional sex is useful in that it helps you adapt more to changing environments and to outpace parasites and things like that to sort of keep your immunity up.
Kat - So, you're kind of mixing your genes up, makes you a bit fitter?
Judith - Yep, exactly. Having obligate sex like we do means that you avoid any sort of inbreeding or you minimise inbreeding so if you can't mate with yourself, you avoid having two copies of a recessive deleterious alleles. In theory, the offspring are fitter, they do better, that's the idea.
Kat - One of the things I'm very interested in your work is the idea of conflict within the sexes. This isn't sort of the battle of the sexes - men versus women. This is kind of conflict between genes at this kind of level. Tell me more about that.
Judith - If you think about it in humans, men and women have - they only differ on the Y chromosome and the Y only has a few dozen genes at most. And so, for 99.99% of genes, they both have them. But yet, men and women look different, they act different, they have different interests, sort of as a sex and so, that means that the optimal function of any gene can differ between men and women. So, it's this idea of conflict over the optimal usage of a given gene.
Kat - So that the genes that you have on your sex chromosomes maybe kind of determine how the other genes get used?
Judith - Well, you can have conflict without sex chromosomes. So, there are lots of animals that have lots of conflict and don't have sex chromosomes at all. Conflict can be in any part of the genome. Any gene that codes a phenotype, some sort of form that differs between males and females. So, if you think about behaviour - so we work on birds a lot, males often find the highest point they can to scream their heads off, so the females notice them. And that's not great if a female does that because she doesn't get any benefit from it. Males don't really take any notice of it and it makes her much more conspicuous to predators. So, the gene that encodes that behaviour is under conflict. So, males want that behaviour, females don't. Males benefit from it, females don't. So, there's this conflict over what it causes.
Kat - Do we know anything about what helps to kind of establish which way you go with it? Is there a role of hormones? Is there a role of the environment, anything?
Judith - Hormones can kind of resolve this conflict in a way. So, if you get one of those genes under the control of a sex hormone, that means that it's only going to be expressed in one sex and that results all the conflicts. So, if they both got the gene, but only one of them uses it.
Kat - What for you is the thing that when you discovered it was like, wow, that's weird!
Judith - That changes by the moment, but I think the thing that I'm most obsessed with currently is the study we just did in turkeys. So, I didn't know about this until I went up and talked to a breeder. So, turkeys actually come in two male phenotypes, two male forms. So, there's this dominant male and then there's this subordinate male, and the dominant male and the subordinate males, they're actually brothers. All the brothers in a given clutch, the winter before they mature get together and they battle it out for who's going to become dominant. The dominant male has the sexually selected traits. It's got this big tail. It's got this iridescent plumage. He makes that really goofy gobbling sound. He has a snood and a wattle and a couple of other things. The subordinate male has most of those, but a little bit less. The subordinate males actually advertise for females with their dominant brother and there's some evidence of having a lot of very attractive subordinate males actually pulls more females in, but they never mate. All the matings go to their dominant brother. They never try and buck the system, so they never and put off their dominant brother. We were interested in the gene expression patterns underlying this.
Kat - Because this is like a wingman basically - well, literally.
Judith - Wingmen, exactly yeah. It's this really bizarre system and I had no idea this was going on. And so, I went up to this breeder and Yorkshire and she showed me the way it works and then I started reading about it and it's amazing. It's also the least tractable system in the world. These things take two years to mature. They're big. They're mean. They're expensive. They don't want people. They're not ideal in that sense, but it's just fantastic. So, we were interested in sort of the gene expression differences between the dominant and subordinate male because both are male. But in some senses, the dominant male is a bit more male in terms of all these traits he's got. It was really amazing for thousands of genes. The subordinate male was a little bit less male in expression and a little bit more female. They only differed from the dominant brothers by a few genes, you know, very much. They're only like 4 or 5 genes that differed a lot, but across the spectrum of all 7,000 expressed genes, they showed these very subtle differences that affect the phenotype, sort of as an aggregate.
Kat - Almost like it's like kind of a third sex for turkeys?
Judith - Not quite, but if you think about sort of sex along an axis, they're clearly on the male side, but they're just a little bit less male. They're not intersex by any means. They can reproduce if they wish.
Kat - If they got the chance.
Judith - Yeah, exactly.
Kat - That was Professor Judith Mank from UCL.

09:54 - Myriad Genetics patent ruling
Myriad Genetics patent ruling
with Nell Barrie
Kat - And so, a big news story that we touched on in last month's podcast was the expected patent ruling on Myriad Genetics about their patterns on the BRCA1 and BRCA2 genes. We were still waiting for a result last month but it's come through. The US Supreme Court has apparently judged that they cannot hold the patents on these genes. They are a work of nature and can't be patented. So, this is broadly good news, yeah?
Nell - Yeah, definitely and I think it's one of those things where people have talked about this for a while and I think to most people who know a little bit about genes, it's always seemed kind of ridiculous that anyone would be allowed to patent something like a gene because it's like saying, I've got a patent on a cat, or if that bird flying there, that's my design and it just seems completely not like a normal kind of patent. So, it's expected but it was almost like, "why did that happen in the first place?" It's a bit strange that that was allowed to happen.
Kat - There were some things that the court said that Myriad could keep. What were they?
Judith - Yeah, so this is about cDNA which is a copy of the DNA. So essentially, if you make an artificial copy of this gene, because you created that in a lab and it hasn't been created by nature, so that cDNA, you can still have a patent on that, is what the Supreme Court said.
Kat - And cDNAs, because they're kind of the gene, but with the bits that aren't important chopped out. I think that's probably quite good for the biotech industry because a lot of the stuff that biotech does is based around these cDNAs,the interesting gene bits. The other thing I noticed was that the supreme court said that Myriad can keep hold of their database too.
Nell - Yeah, this is interesting because I mean, there's so much data out there now around looking at the genetics of different patients, looking at genetics of different diseases and we're increasingly seeing that people want to share, there's much more they want to be able to use everybody else's data for their own research. In a way, that might seem like a bit of a shame, but Myriad certainly would argue that that's where they've invested their money essentially. They put a lot of money into developing the tests that they've made. If we were to say we have to share all these stuff and you can't have patents on anything, there'd be a lot of arguments about how the biotech industry would carry on, where can you get money for investment if you can't make any commercial gain out of it at all? So, in one sense, you could say that this is kind of measured compromise I suppose, but it'll be interesting to see how it all affects genetic testing in the future.
Kat - Myriad have said that they've been doing around 250,000 BRCA tests every year and it has cost around $4,000 and this is just in the US. The situation in the UK and other countries is different. But now, it basically flings open the door for anyone to do genetic sequencing for these genes. So, that's also a hugely good thing.
Nell - Yeah and I mean, I think a lot of people would argue that that's great because you get the competition, you'll get some more innovation around what types of different test companies might come up with. They can improve them in different ways and the other really exciting thing is you can add them to panel tests now because previously, because these tests were patented, you couldn't have them as part of a panel for wider different mutations.
Kat - Which is ridiculous because they're the two key genes involved in breast cancer risk.
Nell - Yeah, exactly and there's also now a lot of big studies happening around the world, looking at different kinds of panel tests, not just for one specific type of cancer, but for genes relating to several different types of cancer in one go. So, it'll be great if we can get to a point where we can sort of share more of this and have a bit more collaboration across all these research efforts and hopefully, this type of ruling will help that to happen better.
13:33 - Three-person IVF
Three-person IVF
with Nell Barrie
Kat - And another ruling that's been in the news this month is that the UK government is pushing ahead with plans to allow doctors to create IVF babies with genetic material from 3 people. But what exactly is the technology about, Nell?
Nell - So, I think one thing the nerds may have objected to is this kind of term of 3 person IVF because it does make it sound like you're getting genetic material from mum and dad and somebody else, mixing altogether and creating some strange...
Kat - Franken baby!
Nell - Yeah, exactly, Franken baby. And actually, the third person is just supplying the mitochondria. So, it's the mitochondrial DNA which is in a lot of ways completely different from the normal genetic material that you'd get in a human cell. So, the mitochondria are there as batteries essentially inside the cell and that was all explained really nicely I thought in a lot of the coverage. It was, you know, what the mitochondria do, how can mitochondria going wrong cause disease, and we heard about that. And essentially, this is about replacing faulty mitochondria that have got something wrong in their genetic material with healthy mitochondria from a donor. The mother and the father's DNA are exactly the same. They mix together in exactly the same ways they would normally and they get a baby that doesn't have a problem with its mitochondria.
Kat - So, that's important to make it clear that it's the mum and the dad who want to have a baby, it's their genes. You're basically using a donor egg to provide the mitochondria that are faulty. It's relatively rare, the kind of diseases that are caused by these faulty mitochondria, but they're absolutely tragic and do affect families in really devastating ways. So, I think it's a very brave move. There is some opposition to it though.
Nell - Yeah, there is and I was having a raid of different people's opinions on this because I find this type of stuff really fascinating, because it's the power of what science can do now and what people feel about that. And it's almost, people have this very sort of visceral emotional reaction to this type of advance and there's all this talk of, we're playing God. We shouldn't be making these decisions, but you're absolutely right. One of the saddest things that came out for me was that some of these mitochondrial disorders, there isn't even a test for them. So, you may know that you're at risk because you've had a baby who's died or a child that's affected, you have another child, and you've got no idea until that disease starts to actually show symptoms whether that child has got a problem or not. So, this must just be really, really tough for parents to go through and they'll sometimes have several children who all die at different stages. There's literally nothing they can do about this.
Kat - And that's being the argument on the science side that if we can do this, we should. And it's not going to be loads and loads of cases. I think it's going to around five to 10 cases every year once it starts possibly by the end of 2014. But it's certainly fascinating example of how building on things like IVF technology that started in the '70s, we can actually make real improvements for public health and hopefully, we'll start to see some babies born in the not-too-distant future. Thanks very for that Nell. That's Nell Barrie, Science Writer.
16:21 - The taste of male fertility
The taste of male fertility
Writing in the journal PNAS, researchers at the Monell Center in the US have made the surprising discovery that two genes involved in taste also play a role in male fertility. While studying mice lacking the genes encoding two taste-signalling proteins, the scientists found they were unable to breed from male animals missing both proteins together, while animals missing just one could produce offspring just fine.
Looking closer, the team found that a drug called clofibrate - which is commonly used to treat diseases such as high cholesterol - could block the human version of one of the proteins, called TAS1R3. But its effects were reversed when the drug was taken away. As well as having implications for rising rates of male infertility in many parts of the world, the researchers also think their finding could also help lead to new types of male contraceptives

17:14 - Cheer up, sleepy genes
Cheer up, sleepy genes
A gene called SIRT1, known to be involved in ageing and metabolism, also plays an important role in sleeping and waking patterns, known as circadian rhythms, researchers at MIT have discovered...
Problems with the body clock have been linked to obesity and disorders such as diabetes, but it's not been clear how it's all connected. Publishing their findings in the journal Cell, Hung-Chun Chang and Lenny Guarente showed that circadian rhythms start to go awry in mice as they get older, and that this can be corrected by giving them more SIRT1.
The research suggests the exciting possibility that drugs that activate SIRT1 could help to keep the body clock ticking as we age, and stave off some of the associated health problems.
Such drugs are already being tested against diabetes, inflammation and other diseases, but they don't cross into the brain, where the body clock's main mechanism is kept. But Guarente thinks it should be possible to design drugs that get round this problem and can help keep the clock going.
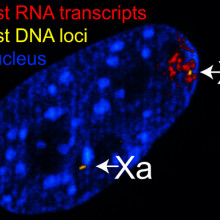
18:26 - Professor Edith Heard - X inactivation
Professor Edith Heard - X inactivation
with Professor Edith Heard
Kat - Now it's time to delve back into the world of sex - specifically, sex chromosomes. In mammals, females have two X chromosomes, while males have an X and a Y. But this double dosage of the X chromosome can cause major problems for the ladies, as Professor Edith Heard, from the Curie Institute in Paris, explained to me.
Edith - The X chromosome is actually fairly large. It actually carries about a thousand genes. So, it's one of the bigger chromosomes at least in humans and mice. The fact that females have two of these and males only have one means that actually, it could cause serious problems in terms of the amount of protein products produced from the female X versus the male X. So, this is an imbalance that is actually intolerable during development and we know that a female that doesn't manage to deal with this imbalance, to have this double dose of the X chromosomes, actually dies very quickly.
Kat - How do females cope with this? What do they do to even out the amount of stuff that they're getting from their X chromosomes?
Edith - In mammals, the way this happens is that one of the two Xs is actually shut down. So, the chromosome stays there, there are two Xs, the DNA is there, but one of the two chromosomes actually becomes transcriptionally silent. So, that means its genes are no longer expressed. The genes are still physically there, but they just are no longer read. They don't produce RNA and they don't produce protein. So basically, you go from a double dose in terms of DNA, but only a single dose in terms of the RNA and the protein that's produced from the female X chromosome or from the female double X chromosome. So, female cells produce the same amount of X-linked RNA and protein as male cells do, and that's how the balance is achieved.
Kat - So they've basically just switched one of them completely off.
Edith - Almost completely. It turns out that there is actually one small part of the X chromosome that is identical to the Y chromosome and it's called the pseudo-autosomal region. So, that little bit of the chromosome actually stays on. We don't know exactly how it manages to avoid this shutting down, but it stays on and it's present in a double dose, but that's okay because males actually have a double dose as well because they have it on their Y and on their X. And there are also actually a few other genes on the X chromosome that aren't in this pseudo autosomal region that seem to escape from this process of shutting down, this X chromosome inactivation process. And again, we're not quite sure why, but we think that for some of them, it could be important that they escape and it might be something that is female specific or maybe XX specific. You need to have a little bit more of some products from the X chromosome in females. But overall, of those 1,000 genes, the overwhelming majority are actually shutdown and this happens very early on in development. Overall, it's actually fairly stable and in somatic cells, once the inactive X is shutdown, its state is very, very stably propagated.
Kat - Explain to me a little bit about how this process actually happens. How do you go from having two X chromosomes in a developing embryo at this very early stage to an adult female, all her cells just have one active X chromosome? What happens? What's the journey?
Edith - So, the journey is complex and we really don't understand it very well, but one of the mammals that we've looked at most or we in general have looked at is the mouse. We think that the way it works in the mouse at least and probably also in humans is that there's a trigger to this process that is this RNA that's called the X-inactive specific transcript (XIST). This RNA is only expressed from the X chromosome that will become shut down. So, this RNA is switched on early on in development and somehow in a female cell, this RNA or the gene that produces it knows that only one copy needs to be switched on. So, it produces this RNA and it's an amazing RNA because it actually coats the whole chromosome. It sort of smothers it and this coating of the chromosome is actually what leads to gene silencing and we don't know how that works. At the molecular level, we actually have no clue how this XIST RNA does its job. But once it's done its job, then it's no longer important because there are other changes that happen at the level of what's called chromatin. So, the DNA of the chromosome that's going to get shut down is wrapped up in proteins and chromatin, the way the rest of the genome is actually. But the chromatin of the X that's going to get shut down starts to change.
It starts to take on new modifications, new flavours we call them, so new proteins associate with it, and we think that these changes actually are what lock in the silent state. So, XIST RNA triggers the process and then these chromatin changes help to keep the silent state silent all through cell division. So, as the cells divide, the X that was shutdown will always be the one that get shutdown. It won't suddenly wake up and remember it should have been active. It actually stays inactive, thanks to these different changes that are mostly at the chromatin level and this is what we call epigenetics. These epigenetic changes are changes that actually allow a stable silencing of a state. In the case of the X, it's almost the whole X. And once this stable silencing has set up, it's the epigenetic marks that allow its propagation through cell divisions.
Kat - This is quite unusual because most RNAs you think, well it's a gene, it becomes active and makes an RNA that goes off and tells the cells to make a protein. So, this is quite weird. Are there any other RNAs that are like this X-inactivation transcript?
Edith - It used to be quite weird. It was discovered over 20 years ago, this XIST RNA. And somehow, it only accumulates over the chromosome that it's produced from and that's a big mystery as well. How does it stay associated in cis as we say, with the chromosome that produces it? So, it did seem to be a very weird kind of RNA, but actually, in the last few years, there's been a whole flurry of discoveries of RNAs that seem to be like XIST. The problem is, we don't actually understand how XIST works and we know even less I think about how these other non-coding RNAs work. And although we can try to make parallels, it's not clear actually whether any two non-coding RNAs or non-protein coding RNAs are doing the same thing or behaving in the same way. So, I think this is a very recent and emerging field of non-coding RNA biology that lots and lots of people are now working on.
But as I said, XIST was discovered over 20 years ago and we still have no clue exactly how it does what it does, and I think that's going to be the situation for many of these non-coding RNAs. So, I think it's very exciting field and lots of people are talking about non-coding RNAs now, but actually, I'm still not sure what they're all doing and I especially don't know what XIST is doing even though I've worked on it for so long.
Kat - That was Professor Edith Heard from the Curie Institute in Paris.

How does the body make fingerprints?
Martha - How does the body make fingerprints? Listener McGraf in South Africa wanted to know whether the same prints get regenerated after an accident like a burn. Eli Sprecher, the Director of the Dermatology Unit at Tel Aviv Soraski Medical Centre says no.
Eli - Fingerprints as you know are characteristic of every individual human being but actually, they are not identical even in identical twins which means that the formation is influenced not only by genetic characteristics of the individual but also by some environmental stimuli. So they actually form as the result of some interaction between the upper part of the sin, which is called the epidermis, and the lower part of the skin which projects into the upper part of the skin and is called the dermis. And when the lower part of the skin is affected by any form of trauma - it might be a burn or treatment with some form of acid - then the signal transmitted from the dermis to the epidermis is lost. And as a consequence the fingerprints are either malformed or do not form at all.
Martha - And what do we know about how fingerprints are formed in development in the first place?
Eli - Now we know very, very little about what are the mechanisms and the signals at the molecular level that are regulating the formation of the fingerprints. Actually one of the only truly significant pieces of knowledge we have about that is coming from the study of a very rare disease where people are actually lacking fingerprints from birth. So by studying this disease it appears that there is one protein that is missing in these individuals, which is called SMARCAD1. And this is actually the only piece of information we have so far about the molecular mechanisms regulating the formation of fingerprints, so it's still quite a mystery.
Martha - That was Eli Sprecher from the Soraski Medical Centre in Tel Aviv.

28:21 - Gene of the month - Antennapedia
Gene of the month - Antennapedia
with Kat Arney
Our gene of the month is Antennapedia, which literally means "antenna feet". As you might have guessed from the name, fruit flies with a faulty version of Antennapedia have an extra pair of legs growing out of their head, where their antenna should be. It's a type of gene known as a homeotic gene, responsible for controlling the overall organisation of body parts like the limbs, wings, head and so on. While fruit flies have just 8 of these crucial genes, mammals have four clusters of related genes, known as HOX genes, with each cluster containing up to 11 genes.
And although mistakes in human HOX genes don't lead to legs growing out of our heads, faults in the genes are responsible for major developmental defects, showing that these crucial genes share common jobs across the whole animal kingdom.










Comments
Add a comment