Fitter fat cells, and Earth spins slightly faster
This week, the cells that vanish when we slim down: are these the link between obesity and health problems like diabetes? Also, the bacteria that might be able to shield us from the “forever chemicals” we’re all eating. Plus, why will 3 days over the next month be a millisecond shorter than they should be?
In this episode
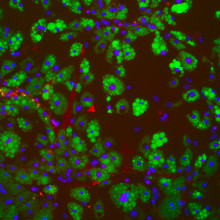
01:05 - Fat tissue gets healthier as we lose weight
Fat tissue gets healthier as we lose weight
Will Scott, MRC Laboratory of Medical Sciences and Imperial College London
What happens to our fat when we lose weight? Scientists in London have just made a surprising discovery. It's not just that fat shrinks, but our bodies also clear out damaged - so called “senescent” - fat cells at the same time. It’s the presence of these cells, they think, that contribute to some of the damaging effects of weight gain - such as diabetes - through producing inflammatory and other abnormal signals. Their removal by the body may well contribute to the improved health status that accompanies weight loss, although what stimulates the disappearance of these cells when we diet - or indeed their persistence when we overeat isn’t yet known. But they might hold the key to better management of obesity and diabetes in future. Here’s Will Scott from the MRC Laboratory of Medical Sciences and Imperial College London…
Will – So we've known for a while that as fat tissue expands and gets bigger, it causes some of the normal roles of fat tissue to not function properly, and that's very intrinsically linked to the complications that people with obesity get. But we've not really understood in great depth why that happens – and even less so for weight loss. We think a lot of these processes may improve when we lose weight, but we don't really know why. And so one of our main mission statements is to understand this.
Chris - And how have you gone about looking at it?
Will - We're very lucky to work in the clinic with patients, and so we've asked some of our patients who are obese or overweight to donate some of their fat tissue to us before they lose weight and then after they lose weight. But also, we've taken healthy, lean people and asked them to do the same. This has allowed us to compare how fat tissues change when we put on weight, but also how they get better when we lose weight.
Chris – And the people that were losing weight, was that just going on a diet and exercise, or were they having other interventions to help them slim down?
Will – So they've had a very specific type of weight loss, which is an operation for weight loss that we call bariatric surgery. It's one of the most potent, or strongest, forms of weight loss, and that's our main reason for studying it. We wanted to see big differences so we could really unpick what was happening – or the biology underneath these things.
Chris – So you get these biopsies from the fat tissue when a person is in their initially overweight state, and then as they lose weight you get a sample again. What do you do with those samples, though? How do you interrogate them to work out what's happening?
Will – We're really lucky to have these amazing tools in our laboratory, where we can take every single cell in a piece of tissue, and all in one test we can look at all of the cells that are there and very deeply look into all of the molecules in those cells. And when we do that across thousands, or even hundreds of thousands of cells, we can build an enormously rich picture of what happens in our tissues when we gain weight and when we lose weight – how cells change, how they communicate with each other, and the problems that they run into that cause these disease processes.
Chris – And what did you see when you did that on the people before and after they lost weight? How do the two compare?
Will – One of the most important things that we see is that when people become obese, some of their cells become stressed and permanently damaged – a process we call senescence. And this senescence process increases as people get more obese. But what was really striking to us is that these damaged, senescent cells seem to disappear almost completely with weight loss. So it looks like the tissue is sort of repairing itself, if you like.
Chris – Do you think those senescent cells, since their number correlates with the risk of having problems from obesity, are the cause – at least in part – of some of the metabolic problems, like diabetes and so on, that people who are obese are at higher risk of developing?
Will – They're one of a number of conspiring factors. Our fat tissues become inflamed when we put on weight, and this is partly because some of the fat cells swell – they get too large, if you like – and they release stress signals. These stress signals cause inflammation, and that process causes some of the fat cells to senesce. But also, senescent cells then release different types of factors that are toxic to the tissue. So you have this sort of vicious circle of processes that all conspire together to harm these tissues.
Chris – And you note that when they lose the weight, these cells disappear. So where do you think they're going? Why are they disappearing?
Will – They're either being eliminated – cells have programmes where they can die, but they do that in a managed way so they don't stress the tissue out – so that's one possibility. The other is that some of the immune cells that are also there in the tissue find these senescent cells and get rid of them. We don't know exactly which is driving this, but what we can see are the key processes within these senescent cells that cause them to be senescent, and to cause other cells to become senescent.
Chris – So if we could find a way to just go in and zap the senescent cells without the person having to lose the weight first, would that also work? If you're right, that should be the case.
Will – That's the principle. Everything has a caveat in science, as you know. Senescence is also important in some contexts. Probably the reason that our cells senesce is for things like wound healing. We damage ourselves, the cells next to the wound senesce, and they release these inflammation and scarring factors, and that helps us heal. But when this is switched on all the time, it causes damage. So we always have to get the right balance with these types of things. There's a lot of interest in trying to treat senescence, but we’ve always got to be careful we do it properly and don’t cause harm at the same time.
07:32 - Gut microbes suck up PFAS forever chemicals
Gut microbes suck up PFAS forever chemicals
Kiran Patil, University of Cambridge
Cambridge scientists have discovered that a certain species of microbe found in the human gut might be able to help defend us against the toxic and long-lasting ‘forever chemicals’ known as PFAS molecules. These substances, originally thought to be harmless and inert are everywhere, including even in some food containers, but they’re increasingly looking likely to be causing hidden health harms. But Kiran Patil, at the University of Cambridge’s MRC Toxicology Unit, has found that a common bowel bug actually seems to be able to soak them up…
Kiran – We wanted to find out if gut bacteria in our bodies can help us get rid of forever chemicals. Their technical name is PFAS, or polyfluorinated alkyl substances. These are widely used chemicals in consumer and industrial products because they're stain-resistant and they're very tough.
Chris – You find them on frying pans and things, don't you? Non-stick coatings. You go to McDonald's and you get your burger wrapped up in a nice sheet of paper that's greaseproof. That's the same family of chemicals, isn't it?
Kiran – Exactly. That's the same family of chemicals. We also find them in waterproof clothing and shoes and so on. And unfortunately, nowadays in our bodies and in our blood.
Chris – But the ubiquity of these chemicals has arisen because of the belief that they are harmless.
Kiran – These chemicals are very inert for chemical reactions. That's why they persist in the environment for hundreds of years. And the initial thinking was that since they are so chemically inert, they were going to be harmless. But unfortunately, that's not the case. There have been many links shown to different diseases, including cancer, reduced fertility, reduced immunity and so on.
Chris – Do we know how they produce those effects, these chemicals? If they are so inert, why should they be having those harmful outcomes?
Kiran – Although they're chemically inert, they're not biophysically inert. So they tend to accumulate inside the body, inside vital organs like the liver and so on. And if they cause inflammation, it can lead to oxidative damage and so on and so forth.
Chris – So what led you down the path of wondering what microbes might be able to do then?
Kiran – The gut microbes have an amazing capability to deal with lots of different challenges. They're already helping us in many different ways, in terms of digesting our nutrients and so on. In the natural environment, they have to deal with lots of chemical stresses. So we wondered whether they would also have some ways to deal with these forever chemicals.
Chris – And what did you do?
Kiran – We started our experiment in a test tube, so to speak. We took a panel of gut bacteria that would represent the average healthy human gut and exposed them to these chemicals to see what they did.
Chris – To distil that then, you took a population of gut bugs that you'd find in the average person's intestine and grew that in a culture, then added these forever chemicals to see what effect it had on the bacteria. Did you add them at the sort of level that, if I were eating a normal diet and going about my life, would be the typical level of exposure?
Kiran – Exactly. So we added many different levels—those matching exposure levels, as well as 10 times lower and hundreds of times higher. We looked into what happens to these chemicals outside the culture, outside the cells of the bacteria. Do they disappear? And if they do, are they broken down or taken inside the cells?
Chris – And what did you see? Do they get into the bacteria?
Kiran – They're not, unfortunately, broken down by bacteria. But we see that the bacteria soak up these chemicals like sponges and store them inside in dense aggregates.
Chris – Really? Do all the bacteria do that? Because you said a mixed population representative of what's in the gut—is it certain types or species of bacteria that do it?
Kiran – Indeed. Mostly Bacteroides, but not all bacteria. For example, gram-positive bacteria tend not to do that.
Chris – Now, obviously a test tube or a culture dish doesn't have an immune system. It's not a lining of an intestine like there would be in real life. So would the same thing happen in a physiological environment—in a gut, for example? Did you test that?
Kiran – Great question. Once we knew that it happens in an in vitro situation, we wanted to know whether it would happen in an animal gut environment. So we did that experiment in mice. We colonised mice with human gut bacteria so that their microbiome resembled that of a human. Then we gave them these chemicals and observed what happened.
Chris – And did the bacteria do the same thing as they did in the dish? Did they soak up these forever chemicals?
Kiran – Yes, exactly. That's what they did. And the way we measured it was by looking at what came out in the faeces. Most bacteria grow inside the gut, but during their regular routine, the mice expel them. And we saw up to a threefold increase in the excretion of these chemicals in mouse faeces.
Chris – Is your argument then that if the bacteria can do this, they are, in a way, protecting us from forever chemical exposure? So I eat some forever chemicals, and I’ve got some of these microbes—at least some of the PFAS molecules will be soaked up by the bacteria, not me?
Kiran – Exactly. That's what our research suggests—that our gut microbes might already be helping us get rid of these chemicals.
Chris – Do you know how the bugs are doing it?
Kiran – We have some molecular insights into this. We now know that there are active molecular pumps that are taking them in, and in some bacteria, actually even pumping them out.
Chris – And just to close the loop on the fact that you're feeding mice these chemicals and arguing that the bacteria appear to be interrupting the flow into the mouse—did you measure the mouse to see whether, when they have these particular bacteria, they end up absorbing a lower dose of these chemicals?
Kiran – Yes. We know that the mice are getting a lower dose because more is pooped out in their faeces. In this particular experiment, we did not measure the mice’s blood content simply because the experiment was set up that way, and our licence only allowed a short-term study. But definitely, in the future, we need to conduct a longitudinal experiment to assess the effect at the whole-body level.
Chris – And what about applications of this? Obviously, it's academically really interesting that this can happen and that you might be able to exploit these bugs in some way. Do you think this is a possible avenue we could use to protect ourselves from exposure to these chemicals?
Kiran – Definitely. I personally see this finding as akin to understanding how cogs and springs work—and once we understand that, we can venture into building a clock. To do that, we co-founded a company called Cambiotics, whose goal is exactly this: to develop probiotics that can be taken orally and, hopefully, help us accelerate the excretion of PFAS from our bodies.
15:00 - Space ice chemical structure cracked
Space ice chemical structure cracked
Michael Davies, UCL and University of Cambridge
Water is one of the most abundant molecules in the universe. Here on Earth we see it in the liquid state, as steam, and also as a solid: ice. In space, though, it’s so cold that it exists mainly just as ice, but it’s not ice as we know it: instead of nice regular crystals like a snowflake, it’s got no structure; the particles exist in a random jumble and this is called “amorphous ice”. But physicists have always struggled to explain why some amorphous ice is of an apparently lower density, until now; because, with a combination of X-ray techniques and computer modelling, Michael Davies has found that the amorphous ice actually contains lots of little crystals: it’s a bit like those chocolate bars that have bits of honeycomb scattered through them. One of the reasons this matters is because it makes the claim that life-giving molecules like simple proteins came to Earth from outer space much less likely; and that’s because the structure of space ice that Michael envisages wouldn’t have much room between the molecules to pack in a cargo of chemical hitchhikers…
Michael – If we think about the atomic structure of something, it's how atoms arrange themselves in three-dimensional space. And if you think of steam from your kettle and water in your glass, it’s completely disordered — that's what it would look like at the atomic level. The atoms just move around with no recognisable pattern. Whereas an ice cube is a solid, and it has a hexagonal structure. If you think of a snowflake, it has hexagonal symmetry. Whereas amorphous ice is kind of like a solid, a bit like the everyday ice you put into your drink. The atomic structure is completely disordered like the liquid — so it’s like a frozen snapshot of the liquid.
Chris – And why should the condition be different on Earth compared to across the universe? How do you know that most of the water out there is in that amorphous form?
Michael – The conditions in space are much harsher. The background temperature, on average, would be around minus 270 degrees Celsius — so extremely, extremely cold. We're talking about things with extremely low energy. So the ice in space — water in space, in nebular clouds (you’ve probably seen photos from the Hubble Telescope, for instance, of these sorts of gas clouds) — will be at very low temperatures and won’t have the energy required to form these nice hexagonal symmetries. The atoms are moving extremely slowly at that low temperature, so they can't rearrange themselves into a neat structure. They're sort of trapped.
Chris – It’s funny, isn’t it, to think that ice needs some heat in order to be “icy” in the atmosphere, in the form we call ice. So why does it matter, then, knowing whether it's in that amorphous state or in the ice cube Earth state?
Michael – If you think of these amorphous solids, they’re basically like a snapshot of the liquid. So to physicists, it’s a very interesting model for liquid water. And now, to get a little bit technical, there are multiple different types of this amorphous ice that we know of. One is low-density amorphous ice. Another is high-density amorphous ice. This idea has given rise to the theory that perhaps liquid water itself is a mixture of these two — a low-density liquid and a high-density liquid. So this is one of the really interesting ways in which amorphous ice feeds into the physics of how we understand something as important as liquid water.
Chris – And how have you been trying to probe this, then? How have you been trying to get underneath whether that is the case?
Michael – In this theory, we want to understand in real detail the structure of low-density amorphous ice. That was our aim — the most common form of ice in the universe. And it's surprisingly difficult to get full atomic-scale resolution so you can literally see each atom and how it’s arranged in three-dimensional space. It’s still a really difficult experiment, even today. In experiments, we use things called X-ray diffraction — like when you go for an X-ray in hospital to take images of materials. And we get back a signal, just a line on a graph. Effectively, we don’t have a full image of the ice. To complement this, where I come in is the computational physics side. This is where I use computers to simulate water. I basically put the laws of physics into the simulation and model water directly. It’s a bit like how you put gravity into a video game so your character jumps up and down in a sensible way. I generated a range of structures — things that are highly crystalline and things that are highly disordered, amorphous. And effectively, we were then trying to find the best match to what we see in experiments from the X-ray data. It was kind of a Goldilocks situation — not too crystalline and not too amorphous. What we found was actually a sort of happy medium: around about 20 per cent crystalline order. So, instead of being completely disordered, this low-density water ice is disordered, but it has very tiny crystals embedded throughout. It’s like it's full of tiny diamonds in the liquid water.
Chris – The stuff that's out there in the bulk of the universe still has some crystalline structure. But, as you say, it’s like a giant mess of small crystals rather than one giant crystal. Why does this matter? Why is it exciting to a physicist to know that that amorphous water out there contains an element of crystalline structure?
Michael – I think there are three aspects. One is how it impacts our understanding of liquid water. Understanding the nature of liquid water is incredibly important for a huge range of reasons, including how we understand life. A second is that this is the most common form of water in space. There are countless ways in which this could impact cosmological phenomena. One interesting example is a theory called panspermia, which suggests that the building blocks of life landed on Earth via a comet. The bulk material of a comet is ice — and it’s actually this amorphous ice. So, if the building blocks of life were to arrive via a comet, you’d have organic material preserved inside the ice. The reason that doesn’t work so well in practice is because crystals in solids can damage cells. A completely disordered solid would actually be a very good transporter for organic materials, compared to the kind of ice we know. But our finding impacts that idea negatively, because the crystals we've found in the material mean it would be a slightly worse carrier for organic material. And perhaps a third point is that it’s just very interesting. We’ve been studying this material for around 90 years, and to find something like this — something that actually changes our understanding of its atomic structure — is really important. Amorphous structures are everywhere in our daily lives. For example, with fibre optics — how you get your broadband — a vital part of that is having no crystalline structure. It’s completely disordered, and that’s how we transport information. So, if a material like water, after 90 years of intensive study, can still surprise us and change how we understand its atomic structure, it raises the question of how many other materials might also hold hidden secrets. And if we could remove the crystals, we might even improve technological materials — potentially making fibre optics even better.

21:53 - Why Earth is turning faster on some days this month
Why Earth is turning faster on some days this month
David Gozzard, University of Western Australia
To time now and this July and August, time will zoom by slightly faster. Earth usually takes 86,400 seconds to do a full spin. But on 9th of July, the 22nd of July, and the 5th of August our planet is expected to pick up the pace and trim a millisecond or so off the day length. Will we even notice? We’ll hear from Ruth Ogden on that score in a minute, but first, what’s actually going on with the planet? David Gozzard is a physicist at the University of Western Australia…
David - This is happening because of the orbit of the Moon. Generally, the orbit of the Moon slows the rotation of the Earth down over time, and this is a gravitational dance between the Earth and the Moon and the Earth's tides. The Moon pulls on the Earth's water and creates the tides, and that creates friction as the Earth rotates under those tides. Then there's this gravitational dance where rotational energy of the Earth is transferred into the orbit of the Moon. So it means we're losing rotational energy and slowing down. Basically, if you wind the clock back 65 million years, the dinosaurs had a much shorter day than we do now. But the Moon's orbit is not a perfect circle, and it's not perfectly aligned with the Earth's equator. So it means the Moon gets closer to the Earth and further from the Earth at different points in its orbit. That means the effect of the Moon slowing us down is reduced. And with various other effects—such as seasonal changes like where water moves or ice builds up on the Earth's surface due to changes of seasons in the northern and southern hemispheres—we get a few days where we rotate very slightly faster than we normally do.
Chris - How on Earth did this get picked up in the first place?
David - So, we model these things—we can model them quite precisely. The Earth–Moon interaction is relatively easy to model. Tides are very predictable. We can see those coming. The less predictable parts are what the Earth itself is doing. So things like, as the Amazon River fills during its wet season or glaciers get more snow on them and get heavier, that shifts where water and ice—and basically Earth's gravity—are distributed across the Earth. So those things we can model to a certain extent. But there are some bits that are trickier to model, such as what the Earth's core is doing—what magma flows are doing beneath our feet. But overall, we do generally have a good model of what these things are doing, and so we can predict in advance that we're going to get this speed-up. So, a 1.5 millisecond shorter day on a particular day—and when we actually come to measure it using stars as a reference, we may find it's close to 1.5 milliseconds, like 1.45 or 1.55.
Chris - Do you expect then that this is a blip effectively on those days, for all the factors and all the reasons you've said, and in the aftermath we'll get that millisecond back? We're not going to all be short-changed a millisecond?
David - Yeah, it's just these three days that are shorter than average. As I said earlier, the Moon is slowing us down steadily. So over time, the Earth's rotation is continuing to slow, and so we all just get longer and longer and longer days.
Chris - Does this make a practical difference? For us humans, I mean, it's neither here nor there, is it? I mean, some days it gets dark earlier because it's cloudier. But there are devices on Earth that are working to fractions of a second. I'm thinking, to take money out from an ATM machine, you're using GPS data because the timing has got to be right. To trade money on the London or the New York Stock Exchange, we're using GPS signals and relativity to get the timing right. So when those sorts of times shift like that, does that have the potential to make differences?
David - Yes—so, not this one event on its own. A single day or a few days being faster or slower by a millisecond isn't going to be noticed by anything other than the people making careful measurements of these things. What happens is, because we're slowing down and we accumulate more and more drift of this time, once we accumulate about 900 milliseconds—so 0.9 of a second—then we add or subtract a leap second. It's a similar concept to a leap year. A year is actually 365 and a quarter days long, so to make sure our calendar doesn't drift out of synchronisation with the Sun over the course of a century or so, we have to add an extra day every four years to account for that quarter of a day. The leap second is a similar concept. Every time we gain about 0.9 of a second, we add on a leap second to our clocks just to keep them in sync with what the heavens are doing.
Chris - Is there a master clock somewhere on Earth, David, to which all of the other clocks—like the satellite clocks and all the other examples you've been talking about—are all synced? And if so, where is that?
David - There's a network of clocks. Various metrology institutes around the world—such as NIST in America, the National Physical Laboratory in the UK, and various others around the world—all have state-of-the-art, very high precision atomic clocks, and these get networked together to eventually calculate a time reference. So there's no one master clock. It is all these clocks working together to create a master, which is UTC.
Chris - And hence, that's what we'd notice when we're a millisecond or so out, and it's against that that we would make the correction.
David - Yes.
Chris - So in your work, sending huge amounts of telescope data over long distances, this is something you would worry about, but for the average Joe like me, I'm not going to notice.
David - Yes. As someone who works in time and frequency measurement and astronomy, leap seconds are a very silly idea—because at a human level, we don't care. These leap seconds are designed to keep our clocks aligned with the heavens. So we're keeping our very high-precision, superior clocks aligned with this old, archaic way of doing things that's far less precise. Why the heck are we doing that? As I said, we have daylight saving time. We have different time zones. We play with the time arbitrarily over a matter of hours as humans. These sorts of precision timing things only matter for our computer networks. So why do we bother adding leap seconds? It would take thousands of years before the leap seconds accumulate to a noticeable, human-scale amount. So we should just do away with these leap seconds and let the Sun and stars drift out of sync with our clocks for a few thousand years—because we're not going to notice. The only people who are going to notice are astronomers. And I think it's better that we update the pointing of the telescope to know, “Okay, the star is actually one second away from where we thought it would be,” rather than getting our high-precision clocks to update and our GPS systems to update and potentially causing various glitches and problems. And so the international timing community has agreed that we're going to stop using leap seconds in 2035. So I think that's a really good idea. I'm looking forward to that.

29:39 - The pressure to be busy in the modern era
The pressure to be busy in the modern era
Ruth Ogden, Liverpool John Moores University
Following on from David Gozzard's explanation as to why Earth is spinning slightly faster this month and next, we thought this seemed like a good opportunity to think about how we humans respond to and register time, and the fact that most of us these days feel chronically short of it. Ruth Ogden is Professor of the Psychology of Time at Liverpool John Moores University…
Ruth - Time is really, really subjective. It's not like a clock would tell you. How you feel about a minute or a second is really heavily dependent on what you're doing and how you're feeling. So we construct time from our activities and our emotions. One of the issues that we face in modern life is that we just don't have enough time. We fill all of our time with a variety of activities. And this can mean that we don't really think about time in the way that we should. We see time as being a resource which should be spent, which should be used up, which can never be wasted. But really, for good well-being and good quality of life, we need to change how we think about time. So I completely agree with David. I mean, the odd millisecond here and there is going to make very little difference, if any difference at all, to the population. But what it does is raise our understanding and our awareness of time. It makes us appreciate that time is something that's important and something that we should take care of.
Chris - Where did we catch this kind of "got to be busy" mindset from? Because I don't remember feeling like I do now when I was little, when I was growing up. And I don't remember my parents feeling that way, but they sure as hell do now, even in retirement.
Ruth - We live in a society where busy-ness and productivity are valued above almost everything else. So you have societal norms telling you to be busy, to always be active, to be constantly doing things. You have smartwatches which bleep to tell you to stand up or to work more, or that you're stressed and need to relax. So we've got this constant stream of pressure to not waste time. And as a result, we seem to have lost the art of doing nothing. We've unlearnt how to relax and how to genuinely have unfilled time. As a result of that, what we often find ourselves doing is trying to relax whilst doing other things—so-called productive relaxation. This might be looking at your smartphone and watching reels of cats or some other thing that you find some basic level of enjoyment in. But the problem we then have is that society tells us this is a bad use of our time, that we should be promoting our intellectual growth or meeting with other people. So I think the way in which we think about time has left us stuck between a rock and a hard place. We're supposed to be constantly busy. But when we need to relax, the things that we do aren't valued. And as a society and as individuals, we really need to think carefully about how we want to spend the time we have and how we can spend it in ways that make us feel good, rather than simply fulfilling society's needs.
Chris - Is there any evidence, Ruth, that the life we are now living in that time-pressured mindset is translating into ill health?
Ruth - There's plenty of evidence that time pressure is bad for all aspects of your health. Time pressure increases stress. It's associated with a greater risk of heart attack. It's associated with risky decision-making, poor mental well-being. It's even associated with greater levels of divorce. And there's some emerging evidence, coming back to what you were just saying about parenting, that time pressure and its impact are intergenerational. So the children of time-pressured parents are themselves likely to feel negative effects as a result of the pressure that their parents are under. For me, this is the public health campaign that needs to be addressed. It's at the root cause of many mental and physical illnesses that are affecting society and individuals today.
Chris - Hard to imagine how we might start to chip away at it, though, isn't it? Because if you think about it, were I going to work 30 years ago, the rate at which I would work would correspond roughly to the rate at which my inbox would fill up, which would correspond to the rate at which the postman would bring the work for the day or someone would arrive at my desk and drop some work off. Now, it's an all-you-can-email buffet of work coming in, distractions coming in, phones pinging, phones ringing. So we've kind of connected ourselves to an artery of incoming pressure that we didn't have before. But the workplace seems to exist and thrive on that. So it's very hard to see how we fix it.
Ruth - This is a really good point. And we can look across to other European countries who are trying to address this through national legislation, such as the right to disconnect, which provides individuals with a legal right not to have to work or attend to their arteries of information beyond their working hours. But these are possibly ineffective because they don't challenge the social norm that busy-ness equals success. What we really need is for employers to have job descriptions and job roles which are the equivalent of one person's day's work. At the moment, we are in a situation where the role and the tasks within a job are vastly greater than the time that people have to fulfil them. And part of the issue here is that if we think back to industrial periods, jobs were based on time. You would go to a factory between the hours of nine to five, and when you left the factory, your job was done. So it was a time-based model. Now we're much more fixed on a task-based model, and the number of tasks continually increases. And it's only when we reduce the number of tasks to be reasonable for one individual to do that we're going to be able to see this breakage in the model we have now and an increase in free time.

35:26 - What is Lyme disease, and how is it diagnosed?
What is Lyme disease, and how is it diagnosed?
Lyme disease is caused by infection with a bacterium known as Borrelia. This parasite can travel through the bloodstream to many parts of our bodies, triggering immune responses and thus a range of symptoms, including skin rashes, fevers, potentially even neurological damage, and more. So how does it get into humans in the first place? James Tytko asked Jack Lambert, professor and consultant in infectious diseases at the Mater Misericordiae University Hospital at University College Dublin...
Jack - To the best of our understanding, you only acquire it from a tick bite. Certain ticks carry the Lyme bacteria, and when they bite you, they inoculate it into your blood and you develop a bacterial infection called Borrelia burgdorferi, commonly known as Lyme disease. Some people get a rash around the site of the bite, and that's the classic bullseye rash. Once the bacteria enters through the skin, it gets into the bloodstream and goes everywhere. So it can present weeks to months after the inoculation as joint pain, cardiac problems, tachycardia, brain issues, nerve problems. It kind of affects almost every tissue in the body. Even studies have shown effects up to a year after the original bite.
James - As many as 5% of ticks in the UK test positive for Borrelia, this remarkable, albeit pernicious, bacterium, which has the ability to proliferate in ticks, mice, deer, our pets, and of course us. It makes humans sick by triggering an immune response and subsequently inflammation in many parts of the body. And it's the varied nature of these symptoms that complicates the next part of your question, Leonard, on diagnosis. But Jack believes there are other factors at play too.
Jack - The issue is that I think in the UK and Ireland, people are led to believe it's a rare disease, you know. In Ireland, they don't keep track of it, so they can make any numbers up—say there's 100 cases a year. I think there are 2,500. For practitioners, it's not on their radar. That's number one. But the next challenge is that the testing is imperfect. So I would say the standard tests that are used by the NHS in Ireland are about 50-50 accurate. So I have people say, “Oh, we did a Lyme test.” You can't have Lyme, the test is negative. But the antibody test, in my opinion, is only about 50% sensitive. So you can have a tick bite, be sick as anything, and have a negative Lyme test. It's Lyme disease. You can have a positive Lyme antibody test from a bite you had 10 years ago. It can stay positive. It's your immunological memory—you can be perfectly healthy and not have Lyme disease. Additionally, if you get the classic rash following a tick bite, that means the infection’s already in your body, going into your bloodstream. You don't need to wait for an antibody test. You just treat it right away.
James - So, Leonard, Lyme disease is acquired by tick bites, which transfer the Borrelia bacteria into our bloodstream. Challenges in diagnosing this disease include the many different symptoms, the imperfect testing, and perhaps a spot of bias in the medical community as to how common Lyme disease really is. If you have a bullseye rash, you should go to your doctor, who should start treating for Lyme disease straight away.










Comments
Add a comment