Is the future bionic?
This week a look at enhancements for future humans: wearable robots, an artificial pancreas, and a replacement retina, as well as limb and head transplants. Plus, in the news, a new hope for global warming, a new therapy to halt MS, what a shock from an electric eel feels like, and how much alcohol remains in food after cooking...
In this episode
00:52 - Gene Therapy Halts MS
Gene Therapy Halts MS
with Brad Hoffman, University of Florida
A potential new therapy to help the 2 million people around the world living with multiple sclerosis, or MS, has been announced by scientists in the US. MS is caused by a rogue population of immune cells attacking a substance in the brain and spinal cord called myelin. Myelin normally works like the insulator that you’d find around an electric cable; in the nervous system it shields and supports nerve fibres. But as the myelin becomes more damaged by the immune assault, nerve signals are no longer faithfully conveyed, and patients become progressively more disabled. Now researcher Brad Hoffman, from the University of Florida, has found a way to reprogramme the immune system to drive the creation of regulatory immune cells that can damp down the action of the rogue cells. Chris Smith heard how, in tests on mice, it stopped the disease in its tracks...
Brad - We do this by taking a harmless virus and engineering that virus to express the same marker that the rogue cells are going after. Doing this liver gene transfer, as we call it, induces a second type of cell called a regulatory t-cell. The regulatory cells can suppress the rogue cells that are causing the damage.
Chris - How are you actually doing these experiments? What are you doing to investigate this, because this is not in patient’s yet is it?
Brad - No, no, no; we’re not in patients as of yet. What we do is we have a mouse model that we can induce a multiple sclerosis-like disease that mimics many of the early stages that would be seen in human MS.
Chris - So talk me through what happens to these mice then and how you set the experiments up to prove that this virus you’ve engineered, which can drive the immune system to regulate itself better, what actually happens when you do this?
Brad - So basically what happens, when we induce disease in these mice, the mice that are not treated would in a process, in about two weeks, in which they become somewhat paralysed. If we give this treatment before we induce disease in the mice, we can completely prevent the disease from even starting. When we do this same technique trying to reverse the disease, about ten days to two weeks afterwards, the mice undergo a reversal of disease and they actually regain function in their extremities quite nicely and almost down to pre-disease levels.
Chris - And you can prove, can you, that the reason that’s happening is because you’ve now got this new population of immune master regulators that are suppressing what were previously these rogue cells attacking the nervous system?
Brad - Absolutely. When we do this technique we can identify the marker we have encoded into these cells that these cells are definitively targeting the same marker as the rogue cell so, when they encounter one another, they will suppress them.
Chris - Is this safe? Because, obviously, you’re putting a virus that shouldn’t be there into liver cells and making them express this nervous system protein that shouldn’t be there, and them making a whole population of immune cells spring up, which weren’t there; a lot of things changing. Are there side effects?
Brad - At this point, based on all the research we have going on, we do not see any side effects at this point. Liver function maintains the mice; are able to live long lives after we have done this if they’ve been treated. We don’t see, at least in the mouse model at this time, any adverse effects.
Chris - How far away do you think you are from being able to embark on a safe human clinical trial because, obviously, for the people who are afflicted with this disease, time is everything because it is a progressive condition?
Brad - We are probably several years away from being in the clinic. Our results are amazing results and we’re very optimistic of this being able to be translated into the clinic. The technique that we have used has already been tested in the clinic in some aspects, but not specifically. So, it’s going to take a little bit of time to kind of merge the technology that’s already in the clinic with the stuff that has to still be qualified for safety reasons to move it along to a human population.
Chris - Considering what might be achieved for people who already have disability, the immune attack on the nervous system often leaves, for want of a better phrase, scarring in the nervous system, which is why people do get progressively worse with MS. So, to what extent do you think you’ll be able to just stop the disease in it’s tracks and to what extent do you think you’ll be able to undo some of the pre-existing disability these people have got?
Brad - That’s, again, a great question. Based on all the research that we are presenting here that we have been working on, the technique that we’re using is definitely more geared towards somebody who has recently diagnosed that’s trying to stop the disease early in it’s path. The idea here is that if we can stop the disease from advancing that the patient would have a much longer quality of life over time.

05:57 - Global warming will take longer than first thought
Global warming will take longer than first thought
with Joeri Rogelj, ETH Zurich
Limiting the increase in global average temperatures to one and a half degrees Celsius above pre-industrial levels was one stated goal of the 2015 Paris Climate Agreement. Many said it was unachievable. But now a new analysis suggests we may have some breathing space. Michael Wheeler spoke to ETH Zurich climate scientist Joeri Rogelj...
Joeri - In the Paris agreement there is a long term temperature goal aiming to keep warming well below 2 degrees, and we also aim at limiting it to 1.5. So, basically, this is annual global mean temperature increase relative to pre-industrial levels, somewhere mid 19th century.
Now the question, of course, arises how can we this? Literature has shown that the warming we see is nearly linearly proportional to the total amount of carbon dioxide that we emit into the atmosphere. That means there is a certain maximum amount of carbon dioxide that we are allowed to emit into the atmosphere ever, also often referred to as a carbon budget.
Michael - So the carbon budget is key to being able to reach that target. What was it that you did to calculate what that carbon budget might be?
Joeri - To figure out how much carbon we can still emit, we basically use three different modeling approaches. One is a simple climate model; another method is a model of intermediate complexity which was used to verify the simple climate model. And finally, we also used results from the most complex, state of the art earth system models.
And in the past, carbon budgets were often calculated relative to pre-industrial so that means that small errors and discrepancies they accumulate over time. So what we did, we basically reset these uncertainties by expressing the carbon budget relative to today. What we find if we take into account where we are today, we get to a budget of around 700 to 800 billion tons of carbon dioxide. This corresponds to roughly 20 years of climate emissions.
Michael - So previous carbon budgets gave us far less time?
Joeri - Exactly. Previous carbon budgets gave us far less time, and basically suggested that keeping warming to below 1.5 was almost a geophysical impossibility.
Michael - An increase in temperature of 1.5 degrees seems like a small increase in temperature. Why are such small increases in temperature so detrimental to our climate?
Joeri - One has to be aware that these increases are global average increases. In general, people are not necessarily interested in average temperature increase. What we are interested in is the risks that can arise with, for example, extreme events. There are clear shifts in the extremes.
Michael - Yes, okay. As the average temperature shifts, so too does the extreme end of the spectrum shift so that we’re experiencing more extreme weather events; is that correct?
Joeri - Yes, that’s correct. In some regions this is a very clear trend.
Michael - Your analysis has given us a larger carbon budget and some more hope, and I think there’s two ways that that message can be taken up. One one hand if people believe that we’ve gone past a point of no return, then they also believe that efforts towards conservation will be meaningless. Your analysis steps us back from that, which I think is a good thing but, on the other hand, it may have the potential to relax people a bit more about climate change. I’m not sure which one of those will be most prevalent; what do you think?
Joeri - Our research really put the possibility of limiting warming to 1.5 degrees back on the table and I think that’s a hopeful message, but that doesn’t really mean that the pressure is off. It still requires actions way beyond the pledges that are currently on the table by countries and global carbon dioxide emissions need to be reduced immediately, to become zero by mid century, and that’s an incredibly challenging feat.

10:49 - Cheap cigarettes contribute to the deaths of young children
Cheap cigarettes contribute to the deaths of young children
with Filippos Filippidis, Imperial College London
Budget cigarettes - that can be as much as 25% cheaper than mainstream brand - are undermining anti-smoking strategies and have contributed to the deaths of thousands of young children and babies across Europe. That's what scientists at Imperial College have found in a recent study looking at 23 European nations, including the UK. Chris Smith spoke with study author Filippos Filippidis…
Filippos - We know very well that increasing tobacco prices reduced consumption so smokers quit or they smoke less than they used to, and young people are less likely to start smoking. We also know that when pregnant women smoke, or when they are exposed to secondhand smoke, and when babies are exposed to it as well, it is more likely that infants will die.
So there is a link between prices of tobacco products, cigarettes in particular, and infant mortality. But what we didn’t know is if differences in prices between average priced cigarettes and cheap cigarettes are important in that sense and whether the ability of cheap cigarettes does have an effect on infant mortality.
Chris - So, if you jack the price up of a packet of cigarettes, then the argument goes, logically, fewer people will buy them because they are more expensive? But, on the other hand, if you got some, for want of a better phrase, cheap and nasty cigarettes which are nonetheless cigarettes, people may switch to those cheap cigarettes and so, in fact, it may undermine some of the benefit of putting the price up?
Filippos - Exactly. The ability of cheap cigarettes doesn’t happen by accident. The market is dominated by a hand full of transnational tobacco companies and they are able to load taxes, for example, onto their premium brands and keep cheap cigarettes at a low price so that poor people, or young people can buy cigarettes and become addicted to nicotine. And then, they hope that they might move onto different, more expensive brands.
Chris -You wanted to know what is the impact of this on infant mortality?
Filippos - Exactly. So we looked into the association between the differential in prices between averaged priced and cheap cigarettes, meaning the difference between minimum and average cigarette prices and infant mortality in the European Union. We found data for 23 European countries on tobacco prices, but also on mortality among babies up to their first birthday, for the period between 2004 and 2014.
Chris - What has changed between 2004 and 2014 in terms of price, and how has infant mortality altered over the same period?
Filippos - This period has seen a decline in infant mortality overall across the EU in essentially all countries. Also prices have increased because of taxation, but the increase in average price cigarettes has been different than minimum priced cigarettes. To be fair, because of high taxation there is less space now than it used to be for tobacco companies to manipulate prices. So the difference between averaged priced and cheap cigarettes was smaller in 2014 compared to 2004 but still, it was as big as 25% in some of the countries that we looked into.
Chris - What is the impact then of a very big differential in the price between if you’ve got a premium brand that’s expensive, but then you’ve got readily available cheap cigarettes sitting alongside it?
Filippos - What happens is that smokers instead of quitting or instead of reducing their consumption, they have the opportunity to switch to these cheaper cigarettes and, therefore, the effect of price increases is attenuated. And what we’ve seen in our study specifically between 2004 and 2014, having a big price differential was associated with more infant deaths than the subsequent year. Taking everything into account, we estimated that if there was no price differential between average priced and cheap cigarettes throughout this period in these 23 European countries, more than 3,000 infant deaths could have been avoided, which is a big number.
Chris - It is, isn’t it? What then is the best strategy for politicians, the taxman, to adopt in order to achieve the best possible outcome?
Filippos - One obvious way is to introduce higher taxation, which some countries like the UK have done. But also, I think governments should look into introducing specific taxes for cheap cigarettes or raising the floor of tobacco prices. I think that would maximise the benefits from increased taxation.
Chris: Filippos Filippidis from Imperial College London. And that work was published in the journal JAMA Paediatrics.

15:49 - Mythconception: microwaves make food radioactive
Mythconception: microwaves make food radioactive
with Izzie Clarke
For this week’s mythconception, Izzie Clarke’s been boiling down the science of microwave ovens...
Izzie - If you’re like me, after a long day you come home starving and the last thing you want to do is spend ages making dinner. Thanks goodness for the trusty microwave oven - the saviour of leftover takeaways. But wait… it’s using microwaves to resurrect my corma and microwaves are radiation. Does this mean, because of my microwave oven, I’m eating a cancer inducing curry?
No, no it doesn’t. So let’s turn up the heat on this mythconception. Let’s start with radiation. It’s all around us in varying amounts and is, essentially, the release of energy. It occurs naturally, but radiation can be given off by everyday items too like from the Sun. Yep, that’s radiation and if you called your mum today radiation was there too. But that doesn’t mean you’re in constant danger. All forms of radiation can be placed within a spectrum - the electromagnetic spectrum. Within this microwaves are fairly low down and, therefore, low in energy.
But how is the food actually heated? When you hit that reheat button, a small wheel-like device, called a magnetron, gets to work. It’s heated core emits electrons that circulate through a constant magnetic field. They seep through small hole-like cavities at a frequency of just under 2.5 billion times a second; this produces a change in field that frazzles our food.
When you nuke your dinner your dinner, it’s actually the water molecules in your food that absorb this thermal energy. It causes them to rotate back and forth and it’s the friction between these molecules that creates heat. Unlike X rays which, in large amounts can be damaging, microwaves are non-ionising radiation and that’s a good thing. This essentially means that whilst the rays have energy, it’s not enough to boot electrons from their atoms and change the chemical structure of your food.
One of the earliest studies into microwave food actually found that no adverse effects were found on the diet cooked by microwaves compared to those cooked conventionally. But be warned, over a ten year period, 21 individuals a day in the US were treated for microwave related injuries, but the majority of cases were for scalding spillages. So, whilst your food won’t be radioactive any time soon, it’s best to pop on some oven gloves before you eagerly took into those leftovers.
Katie - Thank you Izzie, If you have some science you’d like scrutinised, please drop us a line to chris@thenakedscientists.com, you can tweet us @Nakedscientists or find us on facebook and we’ll take a look.
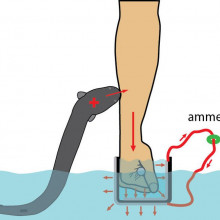
19:14 - The shocking science of electric eels
The shocking science of electric eels
with Ken Catania, Vaderbilt University
In the 1800s, whilst exploring the Amazon, naturalist Alexander von Humboldt documented an attack on a horse by an electric eel, which allegedly leapt out of the water to stun the animal… Yet despite being studied for over 200 years since, this - and a video on youTube were the only documented claims of this behaviour, until, that is, Vanderbilt University’s Ken Catania got his hands on an electric eel: literally. The shock can be ten times the power of a TASER! He told Tom Crawford what - in addition to his own pain threshold - he’s discovered...
Ken -The main function of these high voltage pulses is to activate nerve endings in nearby animals and so, in that sense, it’s a lot like a taser. And then, once you know that, you can start to ask how would you design your taser to creatively activate nerve endings. One thing they can do is freeze up animals for predation; that works very well for fish in water.
But they have this challenge if a predator was to come at them in shallow water which actually happens in the Amazon during the dry season. How would you defend yourself against a predatory cat, or some other predator, or a crocodilian that was coming at you and you only had a limited amount of electric resources to deliver? Well, it turns out the best way to do that without having much of the energy dispersed in the water is to lift out of the water, press against the threat directly, and give off you high voltage. It’s really a shocking behaviour!
Tom - Nice, you beat me to the pun. So just to re-clarify what you’ve said, these electric eels, they literally jump out of the water and then zap their target?
Ken - Exactly. It started by realising when I approached them with a metal rimmed net, they would attack the net by leaping up and shocking it, and that’s the only time I ever saw an electric eel try to come out of the water. So it was a big clue that there’s something special going on with these conductive nets. The eels interpret conductors as living things; they interpret large conductors as threatening living things, and this is clearly a defensive behaviour.
So the research that’s recently published was basically trying to work out the puzzle of the electric circuit that develops as the eel comes up out of the water. And so, I had designed a series of experiments to address each variable in that except for one, and that was the target or, in this case, my arm.
Tom - Sorry Ken, let’s just back up a second. You were purposefully putting your arm into the tank with these electric eels in order to be shocked?
Ken - Eventually… yes. But I got there by really wanting to know the answer to each of the variables in the circuit. I wanted to know the electric motor force of the eel, the internal resistance of the eel, the resistance of the water, and then the variable values of this other resistance that develops as the eel comes out of the water, and the sum of the current can pass back down the eel to the water. I had solved for all of those and it gave the illusion that wow, I’ve really got this puzzle figured out. But there’s a quirk of electric circuitry that if you have two resistors in parallel, which is something that happens when the eel comes out, you need to know both of those resistances to understand how much current flows in the circuit. I was scratching my head about what am I going to do about this? And the final answer was to develop this device that could measure the current through my arm.
Tom - I love how you said there “the obvious answer” was to electrocute yourself.
Ken - Hey, who doesn’t want to get electrocuted by an electric eel? Come on, deep down. I should also say this is quite a small electric eel so I wasn’t taking any crazy risks. You know, Faraday played with electric eels; Humboldt played with electric eels; there’s a long history of people experimenting with this so-called “hands on” approach. I mean, I wouldn’t do it lightly with a large eel, and I didn’t do it with a large eel.
Tom - You’re saying you were trying to measure all of the variables in the system to be able to calculate the electric circuit, so what was it that you found out?
Ken - Yes. The voltage of the small eel that I was working with was about 200 volts. Eels can get up to 500, sometimes 600 volts when they’re very large and they can get to a couple of metres long. I guess one of the key things that I think this research allows you to do is pretty easily plug in the numbers to extrapolate to these larger animals and we know that people get shocked in the Amazon by this behaviour. In fact, there’s a kind of amazing video that went viral recently showing a fisherman getting shocked by a large electric eel with this leaping behaviour.
Now we have a good idea of how much current goes through a person based on eel size. Specifically, for my case, it was about 40 to 50 milliamps and that was sufficient to be quite a deterrent -I’ll put it that way. If you’ve gone to a really colourful, interesting party and have been tased recently, it would be a lot like that. It would be, for a large eel, it would be 20 times the power of a TASER.
Tom - Oh wow!
Ken - Yeah. It was a lot of fun to do - if you can believe that!

25:02 - In the future, will we wear robots?
In the future, will we wear robots?
with Russ Angold, Ekso Bionics
Before we go inside the body, what about devices that assist from outside? Katie Haylor spoke with Russ Angold, co-founder of Ekso Bionics, a company that makes robotic exoskeletons you can wear to help you lift heavy things...
Russ - It’s just like it sounds; it’s an exoskeleton that goes around the person and looks like a robot. It has actuators very similar to robots that help that user move, or provide assistance.
Katie - I’m imagining a kind of Transformers/Independence Day type thing here. Is that right; it's that scifi?
Russ - It’s probably not like a transformer - it’s slimmer, smaller. We try to make them as light and as svelte as possible so that users can move around the environment without bumping into things.
Katie - Okay. So how do they actually work then?
Russ - You just put it on. It depends on what type of exoskeleton it is. We have medical exos that are designed for people with stroke or spinal injury; they get those sized and put on during our rehab session. We have other exoskeletons like the Ekso Vest that you put on like a backpack and strapped to your arms and your torso, and it provides assistance to your arms. So it depends on the type of exoskeleton you’re wearing.
Katie - I guess the medical side is a little bit more obvious in terms of who might benefit from this but what about the other side - just lifting heavy things? What’s the demand for this sort of technology?
Russ - We actually, about 2 years ago, started getting a lot of interest from construction companies, and industrial companies, manufacturing companies, saying that they have an ageing workforce. They know how to do the jobs but their bodies are just breaking down all the time and is there something we can do with exoskeletons to provide them that extra endurance they need to get through the day safely and productively?
Katie - How are these sorts of things powered then?
Russ - That’s a really interesting question. Some of our early devices had no power at all; basically, what we do is cancel out the effects of gravity. You can imagine I have this vision of everyone that’s hung a ceiling fan and tried to hold that ceiling fan up in their hands, really what you’re fighting there is gravity. We have our EksoVests that just uses spring to cancel the effects of gravity and basically make your arms weightless so you can hold your arms out forever without getting tired, and that’s step one.
Eventually they’ll be powered to provide more capability. One of the big applications we see is material handling. If you think of guys moving object around, so your luggage handling, your construction worker, your maintenance worker manufacturing. People are always moving stuff and we think that’s a great application for exoskeletons.
Katie - This seems to mean then a lot less strain, a lot less potential backache, aches and pains, does this have any consequence for productivity - are people able to do manual jobs quicker?
Russ - Oh absolutely. There are able to do the job quicker and safer. We had an example where a guy was drilling anchors overhead with a concrete rotary hammer, and without our zero-G arm was drilling about 80 holes a day. We gave them the zero-G arm, which then makes that tool effectively weightless and helps push into the ceiling and he went from 80 holes a day to over 400 holes a day. And he was able to go home at the end of the day and help his family with the farm and not be completely exhausted from a long day’s work.
Katie - Wow. That is an impressive difference. You mentioned there the zero-G arm, and I know you have a couple of other products, one being the EksoVest. Maybe you can tell us a little bit about the difference between those?
Russ - Sure. The zero-G arm basically amounts to a worker’s aerial lift - platform or scaffolding - and connects to their tool and makes that tool weightless. Again, it’s not powered but it’s cancelling gravity, but if anyone tried to hold a 20 pound or 10 kilo tool in front of them for any period of time, it’s exhausting. We basically made that weightless so the user can focus on the quality of work and getting the job done without straining their arms.
The vest is a wearable device. It actually straps to the wearer and makes their arms weightless so you think of automotive assembly, aero assembly in factories, anytime where you’re spending a lot of time with your hands over the head. Which happens a lot in the construction world now because a lot of the systems are put in the ceiling. It makes your arms buoyant like you’re just floating under water and relieving all that stress and strain from you shoulders and back.
Katie - Is precision also a factor here; it’s something that is particularly important in construction as well as just productivity?
Russ - Right. Well you can imagine, once the strain of either holding the heavy tool up or the fatigue of holding your arms up is gone, then all of a sudden we see an increase in quality which means less rework, the job gets done better and you don’t have to come back and finish things off, so quality is a big asset. We see safety, productivity, and quality as the three real benefits of using this type of technology.
Katie - Very briefly, are we in danger of all turning into couch potatoes as a result of this kind of technology?
Russ - Not at all. This technology gives you more endurance, but the workers are still working or using other muscles that we can’t augment, so there is no risk of that...
30:29 - Artificial pancreas treats type one diabetes
Artificial pancreas treats type one diabetes
with Joan Taylor, De Montfort University
Heading inside the body now. About 40 million people worldwide have what’s called “type 1 diabetes”. These individuals cannot make the sugar-controlling hormone insulin, usually because their immune systems have attacked the tissue in the pancreas that normally produces this hormone. As a result, the body cannot regulate blood glucose levels itself and the person has to regularly measure their blood sugar and then inject artificial insulin to keep their glucose levels stable. This can be inconvenient, uncomfortable and embarrassing. But if they don’t control blood sugar they risk damaging their eyes, kidneys and blood vessels. So, there is a strong motivation to keep them in check. Chris Smith heard how, to solve this problem, Joan Taylor at De Montfort University has built a wrist-watch-sized implantable artificial pancreas, with no moving or electrical parts, that just uses a clever glucose-sensitive gel packed with insulin to do the job...
Joan - At the present time we’re looking at three implant sites. They’re all inside the body cavity near to the liver with blood vessel access and that’s really important because our real pancreases secrete insulin into the blood supply to the liver because it’s the liver that does the chemistry; it’s just that the pancreas is the supplier of the drug. We want to try and mimic that so we had an idea that we would produce a chemical substance that was responsive to glucose.
So we actually synthesised a polymer which is, in our case, a kind of semi solid plastic and that substance contains a glucose receptor and a glucose interactor so that the interactor makes contact with the receptor and can form a lock and key which unlocks when you add glucose to it. As glucose floods the polymer, then the polymer becomes much more permeable. What we did was to build that into a device that holds insulin, so that the insulin is forced to exit through this polymer to reach body fluids where the same polymer senses whether the glucose is high or low and then transmits the insulin either fast or slowly, depending on the need that it discovers on contact with body fluid. It’s completely different, it’s not electronic and it’s not biological so, because of that, it is actually very fast acting, it doesn’t have delays. And, because it’s not biological, it doesn’t need immunosuppression drugs to be given which is the case with transplanted pancreases.
Chris - How far have you got with this so far; is this just in a dish or are you actually doing this in animals now?
Joan - We did a lot of study over the last 20 years in dishes but it got to the point where my mentor of many years said to me “do you know, if you’re really saying this could be done, you’ve actually got to prove it.” It occurred to us that there was really no way of proving this with cells in a dish because what you need here is the device to respond to the biological system in its diabetic state, and that animal or volunteer to respond back. It’s an interactive system so you’ve got to do it in a live system. So what we’ve done is to use diabetic pigs and that work has really pleased us. We’re doing well with that.
Chris - So you have a pig with diabetes, you implant one of these devices - what happens to the pig?
Joan - The pig is quite happy; it doesn’t feel it. The device itself is about the size of a pocket watch and once it has recovered from the implant, we watch the blood sugar come down to normal levels. Then what we do is to challenge the pig’s diabetic system by giving it large amounts of treats and watching how well the hypoglycemia is controlled. The definition of being not diabetic is that a large burden of glucose should normalise within two hours and, in our experience, we have had the normalisation happening in less than an hour.
Chris - How much insulin can the device hold? In other words, between top ups, how long would a potential diabetic be controlled for?
Joan - In terms of actual volume it’s about 5 millilitres, but in terms of the time that would control symptoms for is about 50 days.
Chris - I suppose one risk is that one is walking around with, locked away inside this stuff, potentially a life threatening reservoir of insulin?
Joan - You are right there. That’s an inherent problem with the design is it presently exists but we have plans to modify our system so that instead of the insulin reservoir being liquid, it itself will be in gel form. So, should the device break, then there wouldn’t be an instant leakage of the insulin.
Chris - How long will the device potentially function for? In other words, how long have you taken it out trying this to know that the polymer doesn’t degrade, it doesn’t break down and does reliably control blood sugar?
Joan - We have kept the gel in a simulated animal fluid for two years and it hasn’t degraded. What we did was to put it inside a membrane bag and that bag has pores in it that do not allow enzymes into the gel that would degrade it. It doesn’t allow bacteria in that would degrade it but it is large enough to let insulin out. So, based on that experiment, we think that the longevity of the gel is at least two years and because the gel is in good shape when we’ve taken that experiment apart again, we think it should be much longer too.
Chris - In terms of the pathway into the clinic, how far along that road are you?
Joan - You have to have a statistical number of animal experiments to show that the treatment is safe and effective and once we’ve finished our small number of experiments we will have to apply for grants to do that larger number. And then, once you have that evidence, you might be allowed to take it into phase one clinical trials, so I would think there’s five years work there. Then, once you’d got into phase one, there another similarly long period before it’s actually released onto the public in a bigger way so quite a long time I think. But what we hope is that we’ll be able to show that this thing actually works and, even if we don’t take it further, it will add to the knowledge out there, and that if we don’t then somebody will take this out into clinic. It’s advantages for diabetic sufferers are that not only does it keep the blood glucose steady, which is a major advantage, but that the whole thing is invisible from the outside and, really, what we just need now is the facility to develop what we’ve got.

38.19 - Transplants: from hands to heads
Transplants: from hands to heads
with Simon Kay, Leeds University
We’ve considered robotic exoskeletons, and an implantable replacement pancreas, but sometimes a prosthesis isn’t right for a range of reasons and a transplant of human tissue is the best option. One example is when a person loses a hand or a limb. But not only are there psychological obstacles to overcome with this sort of surgery, practitioners were initially also worried about the challenge posed by immune rejection. For this reason it’s only within the last 20 years that doctors have carried out the first hand transplant. Now it’s gathering pace, and Alexandra Ashcroft heard how, from Leeds University hand surgeon Simon Kay, who is also Director of the UK Hand Transplant Programme...
Simon - When you lose an upper limb, or part of an upper limb, the options are to live with what you have, to create a prosthetic, or to have a limb transplant, and limb transplantation is very new. You take a hand from a recently deceased person who’s on the donor register, just as you would for a kidney or a liver, and you transplant that onto the limb that’s deficient. But then that limb lives and has nourishment from its blood supply and its function begins as the nerve regeneration occurs in the limb.
Alex - You said that it’s quite novel, what sort of surgical advances have you and others made to get to the point where you can transplant limbs or hands to other people?
Simon - The first hand transplant took place in ‘99, and the most important hurdle that we didn’t appreciate in the very early days is the behavioural one or the psychology of the patient. The other main hurdle that was much vaunted but was actually proved to be very easy to deal with is the immunological rejection risk. That is to say, just as you can reject a kidney or a liver, you can reject the limb. So that barrier that we thought was enormous has actually begun to recede. The great thing about a limb transplant that has skin is that when it begins to reject you can see it immediately as a rash, so we found that the issue of immunological rejection is relatively straightforward to deal with. The other barrier has been understanding whether the long term regeneration of the nerves, and the recovery of movement and feeling in the limb is going to be at level that we would think is useful and, I’m pleased to say, that’s proved to exceed our expectations as well.
Alex - Are donors a problem?
Simon - Nobody really cares what their liver or their kidney looks like as long as it does the job. In hand transplant it really is important because one of the functions of the hand is that it’s on view all the time. Although we have a small volume of recipients in the UK and a large volume of donors matching, not just immunologically but also in size, approximate age, and skin characteristics makes the pool much smaller, so finding the right donor is a challenge.
Alex - How do these limb transplants in terms of the outcomes that they achieved, how do they compare to people who’ve had prosthetics?
Simon - Comparing transplanted limbs with prosthetic limbs is very interesting. They’ve been, as it were, in competition between transplant and prosthesis. In our view that’s not appropriate because they do very different things. The vast majority of patients will be improved and functionally improved by prosthesis alone. The great benefit of a prosthesis is that you don’t need to give the patient drugs and immunosuppression in order to use it. However, the limitation of a prosthesis is that it’s not human and one of the things about the hand is that it’s one of the very defining attributes of a human being. So it’s warm and sensate; we gesture with it, we stroke with it, we feel with it; that humanity you won’t get from a prosthesis. You do get it from a hand transplant; you get the cosmetic, the aesthetic, and the functional, and also the humanness of it, but it is at the cost of immunosuppressive drugs.
Alex - And they have pretty severe side effects I understand?
Simon - I think rather than saying they have severe side effects, they have significant side effects which can, because we now have a great choice of drugs, we can chop and change, mix and minimise the side effects. But, there’s not doubt that for a young person having a hand transplant, if we assume they keep that transplant for the rest of their natural life and they’re on immunosuppression for the rest of their natural life, that life will be slightly shortened. So when you’re contemplating a hand transplant, just as with any other major medical decision about treatment, you have to weigh the benefits of the treatment very carefully against the disadvantages. And, because we haven’t done hand transplants until ‘99, we’re still beginning to fill in the boxes on either side of that risk/benefit equation.
Alex - Where do you think your field will be in the next decade to come?
Simon - I think the proof of concept is now virtually done. These things move in a slow pace, but I think it is becoming understood and accepted that hand transplantation is reliable. And, furthermore, that it produces very good and useful results that are enduring and those have been very important bridges to cross.
Alex - Shifting the focus a little bit to Sergio Canavero’s first attempt to do the World’s first human head transplant in December; what do you think about this?
Simon - I think it’s a thing that needs to be debated and the ethics very carefully considered. But when we’re talking about head transplant, what we’re really talking about is a body transplant. The internal human being resides within the cranium and if you have an alert, active human brain and you have a body that is failing then there is a logic to saying well, can I replace the body? And there’s no theoretical reason why you can’t. So, if you had a quadriplegic with organ failure, what you might say is well, the body is an inert support mechanism for the brain and I’d like to exchange that. Of course, it’s conceptually a head transplant because that’s a smaller part. But I see no overriding technical reason this can’t be conducted as long as you’re not expecting the body to function mechanically and get up and walk.
Alex - Because of connecting the spinal cord?
Simon - Because connecting the spinal cord is the “holy grail” of spinal surgery and that’s nowhere near at the moment...
Why do immunosuppressive drugs harm health and shorten life?
Chris Smith answered the question...
The reason is that the immune system is there for a reason. And if you have to take drugs to deactivate the immune system, they don’t just deactivate the bit of the immune system that is potentially attacking the new organ or the graft, they suppress the entire immune system. This means that you’re at a greater risk of getting infections from various sources: bacterial sources, viral sources, fungal sources; so that can have an impact.
But, also your immune cells patrol your body looking for cancer and there is, therefore, a higher risk that some of your cells may become cancerous and not be removed by the immune system because it’s being deactivated.
There’s also a third thing which is that the drugs themselves are poisonous. Every drug that we take can has side effects, and so, if you take a drug into your body, there will be side effects and some of these drugs do themselves have damaging effects on end organs including your kidney, for instance, and the liver, sometimes. As a result of that, that can also render you slightly less healthy.
So all these things add together to a slight reduction in health. But, of course, that’s tiny in proportion to the enormous benefit returned to you by having had a transplant of, say, a heart, or a lung, or a liver or, in this case, we’re talking about a limb...

46:26 - Retinal Implant Restores Sight in Retinitis Pigmentosa
Retinal Implant Restores Sight in Retinitis Pigmentosa
with Robert Maclaren, University of Oxford
Now let’s take a look at what modern science can do for a growing problem worldwide, which is blindness. As more people live for longer into old age, more of them are succumbing to sight problems, often because of disease processes affecting the retina, which is the patchwork of light-sensitive tissue at the back of the eye that converts light waves into brain waves and enables us to see. If this is damaged it cannot repair itself and we suffer sight loss. Speaking with Chris Smith, Oxford University’s Robert MacLaren is working with a prosthetic retina, which is currently in clinical trials...
Robert - We're working with a German engineering firm, Retina Implant, and I should say that the development of the technology is really something that’s been ongoing with Retina Implant for the last ten years. We’ve been working with them over the last five years in clinical trials in Oxford. The purpose of the retina implant is to put at the back of the eye a light sensitive array that is electronic rather than biological.
If I can explain it another way - the light sensing cells known as photoreceptors gradually degenerate in diseases like age-related macular degeneration and retinitis pigmentosa. The electronic retina is light sensitive, a bit like your mobile phone with a digital camera, it senses the images and then it stimulates the retina electronically in place of the photoreceptors.
Chris - The retina has multiple layers and in one place are the photoreceptors - the rods and cones. They send electrical messages to cells further down the retina, which is what makes the optic nerve and sends these messages pinging off to the brain. So, in the diseases that you’re looking at, those rods and cones are gone, but the cells that make the optic nerve and can send messages to the brain, they still survive and you’re putting signals into those cells?
Robert - Yes, that’s absolutely correct. The pathology of retinitis pigmentosa is loss of one layer of the cells - the light sensing cells. Of course, if someone’s lost these cells, they are unable to perceive light at all and they’re completely blind, but the eye itself remains relatively intact. The eye looks normal, it moves around, it focuses the image and, most importantly, the optic nerve which connects the eye to the brain is also relatively normal.
So the technology that we’ve been looking at with the electronic retina is really for patients with retinitis pigmentosa. It wouldn’t, for instance, be suitable for patients who have a disease known as glaucoma in which the nerve is damaged or, indeed, if the eye had been lost through an injury or trauma.
Chris - How big is the device and how do you, as an ophthalmic surgeon, get it inside someone’s eye?
Robert - It’s approximately three and a half by four millimetres in size and that’s the actual light sensing part which has got 1,600 pixels on it. There’s a flat cable as well that connects it to a power supply, and the power supply is located under the skin behind the ear. So there is a cable which goes underneath the skin, across the side of the head under a muscle known as the temporalis muscle to connect it up to the power supply.
We have to slide it under the retina without damaging the retina. We do that by sliding it through a very small slit in the wall of the eye. It is quite fiddly, I have to say; there’s a lot of connections and things, and placement to do, and it takes a long time to do that. But, as ophthalmologists we are quite used to working in small spaces so it’s relatively straightforward in terms of adapting the technology we already have.
Chris - A person who has it implanted and then you switch it on, what is their experience?
Robert - Well, this is quite an amazing time for us all actually, when we get someone who’s been blind for maybe ten or even more years, who has no light perception to then sit there in that room when we activate the device for the first time, usually about three weeks after surgery, enough time for things to heal up. For them it’s a life changing moment and it’s very exciting for us. Everyone in the team gets to know the patients well, and we’re all very anxious until that final switch on takes place and once the patient’s able to see things we know that the whole process has been a success.
Chris - Do they see colour or at the moment are you just seeing spots of white light on a black background?
Robert - Yes, it’s very much black and white. The light sensing cells - photoreceptors - come in three colours effectively, red, green, and blue and they build up a coloured image but that processing we don’t currently have in the implant - it’s really just on or off. Sort of similar to the very early television pictures, a very grainy black and white image coming and going. It’s certainly nothing that you would describe as normal vision, but it’s enough for these patients to get around and see things and have some independence.
Chris - Can they, for instance, recognise a face? Because people who have visual decrements say that they really struggle to see people’s faces across the street; they can’t read their favourite book; they can’t watch their favourite television programme. What level of function do they get with this device?
Robert - Well, that’s very interesting because, of course, the human brain is an amazing thing. And whilst patients wouldn’t recognise a face on a still image, for instance looking at a photograph, when the image is moving around they get a lot more information out of it and they can see and recognise people by the way they move. Also, I know some of my patients have described being able to recognise different dogs, for instance. Of course, a lot of blind patients have guide dogs so there are cues there. But the moving image is the most important and that gives them the cues they need to recognise things in more detail than you would think based on the number of pixels.

52.10 - How much alcohol does cooking remove?
How much alcohol does cooking remove?
Alex - I asked Vayu Maini Rekdal from Harvard University to turn up the heat on Zettie’s question…
Vayu - Alcohol containing ingredients are really prized for their ability to impart complex flavours and aroma. Many people when they cook with alcohol actually assume that cooking completely evaporates the alcohol because the alcohol has a lower boiling point than water. But, contrary to popular belief, a significant amount of alcohol could actually remain at the end of the cooking process. This is important to consider when you serve dishes to people who may need to control their alcohol intake.
Alex - Wait… so my coq au vin can still get me drunk? How do we know this?
Vayu - Most of what we know about cooking with booze comes from a 1992 study in which researchers measured the alcohol levels at the end of cooking a number of different dishes including orange chicken, and scallop oysters. Each recipe represented a different way that we might add alcohol to cooking - quick flaming to simmering food for many hours. They found that, in general, the final alcohol percentage depends on time, on temperature, the starting concentration of alcohol, and the size of the pan.
Alex - So which culinary techniques are the most effective at purging alcohol?
Valumini - Flaming a dish retained 78% of the starting alcohol, contrary to the belief that flaming would burn away all of the alcohol. Simmering for 10 minutes removed only 40% of the alcohol but if you simmered for longer, say 2½ hours, it got rid of 95%. So those worried about their alcohol intake should maybe consider slow cooking.
Alex - Any other tips for those looking to avoid getting “sauced” on “sauce?”
Viyu - Well, with simmering, the final percentage depended not only on the time but also on the surface area of the pan. A wider pan removes way more alcohol. But, in general, while the amounts of alcohol in dishes is usually very low, predicting the final percentage in your dish is not a simple task, which also means it’s hard to know how recovering alcoholics will respond to different dishes with alcohol. However, it’s overall safe to say that the perception that alcohol is removed with cooking is a complete myth Science tells a different story.
Alex - So, if you’re cooking for a teetotaller, best to play it safe and keep the wine for another time. Thanks to Vayu Maini Rekdal for serving that up.
Next time: we’ll be buzzing about John’s question...
We know that flies process movement much quicker than humans, which is why it’s really hard to swat them. But is it true that if you move slow enough then the fly will not register the movement and, therefore, you can actually get it...?
Related Content
- Previous Algal proteins may boost crop yields
- Next How do tissues grow?










Comments
Add a comment