From Stem Cells to Brain Cells
We speak to scientists turning embryonic cells into nerve cells to treat Parkinson's Disease, and growing an interconnected system of organs on chips in the lab. Plus, how antibiotics taken during pregnancy may affect an infant's behaviour, and why air travel is about to get a lot bumpier...
In this episode

00:53 - Antibiotics during pregnancy may affect a child's behaviour
Antibiotics during pregnancy may affect a child's behaviour
with Dr John Bienenstock, McMaster University
Penicillin has saved millions of lives since its antibacterial properties were realised in patients in 1942. Now, literally thousands of tonnes of antibiotics like penicillin get used every year around the world. But could there be consequences for a developing baby’s brain if its mother takes antibiotics during her pregnancy? New research from Canada, carried out on mice, suggests that taking antibiotics changes the spectrum of bugs in the mother’s - and subsequently the infant’s - intestines. John Bienenstock told Chris Smith how this might affect brain chemistry and the behaviour of the infant after it is born...
John - We postulated an antibiotic might change the balance of bacteria in the gut. If we gave it, in pregnancy, and then continued until the animals separated and were independent from their mothers, would there be a change in brain activity or behaviour? And the short answer is yes - animals became aggressive, quite unusually, and there were chemical changes in the brain. These are associated with the changes in the bacteria in the gut.
Chris - How do you know, though, that the differences you’re seeing are not just a side effect of the antibiotic molecule?
John - What we did was to give another group that were receiving antibiotics a bacterium commonly referred to as a "probiotic", and when we did that we found that there was a marked difference. The effects were not so significant or concerning in the brain and in terms of behaviour, and the changes that we saw in the bacteria in the gut, which were drastically altered by the antibiotic, were actually not seen. So we take this as demonstrating that the bacteria may well be significantly involved in the causation of the abnormalities.
Chris - If you look in the brains of the mice that you have treated in this way and seen these changes and behaviour, are there any obvious differences that would account for why they’re behaving differently?
John - Yes. When we do, we find particular changes, which are quite dramatic, in the frontal cortex which is part of the brain that tends to regulate emotions and reactions in that sense. Those changes are associated with a particular molecule or receptor which has been shown to be associated with aggression, so our thought is that those are, in fact, associated with these behavioural changes.
Chris - Is this potentially then irreversible? Are the mice - once they’re like this - are they locked into this altered behavioural state?
John - We don’t know the answer to that. It’s a key question because, obviously, what we’re talking about is the difference between prevention and treatment. In addition, we don’t know whether the effects that we see are only related to pregnancy or whether, in fact, separating the experiments into two halves, one in which we treat animals with antibiotic in pregnancy and another which treats only the pups and then looks at the long term at offspring, which one of these is going to have that effect. We suspect that the results will be that the effect of the antibiotic is in pregnancy.
Chris - To what extent do you think that - because this is a study in mice isn’t it - what extent do you think this is relevant to humans?
John - It’s the most important question. In North America, currently, the statistics show that every child by the age of two receives one or two courses of antibiotic. That doesn’t mean to say that the antibiotic isn’t necessary - it’s just a fact. We know very little about the incidence of psychiatric disease, at least the risk for the development of psychiatric disease, as a result of antibiotics. We do know that antibiotics are associated with several different clinical diseases such as inflammatory bowel disease, colon cancer and also, interestingly enough, obesity. But we do not know whether antibiotics in pregnancy have any affect at all, so the epidemiological evidence will be some time in the coming to either prove or disprove the current results in mice.

05:29 - Climate Change will increase Turbulence on Flights
Climate Change will increase Turbulence on Flights
with Dr Paul Williams, University of Reading
We’ve seen many recent extreme weather events - from mudslides in Columbia to flooding in Australia - which scientists say are a consequence of climate change; but it’s not just the weather that is affected. The Earth’s atmosphere is made up of several layers of air which all flow around each other in patterns known as jet streams and an increase in temperature will cause these to speed up. This is bad news for air passengers, including the 1 million people currently airborne at this very instant, because an increase in the speed of the jet streams will cause more turbulence making flying less comfortable and potentially more dangerous. Tom Crawford heard how it’s happening from atmospheric scientist Paul Williams…
Paul - We’ve been looking at turbulence over the Atlantic Ocean, and specifically severe turbulence which is strong enough to hospitalise people and, indeed, it does cause many serious injuries every year. What we’ve been interested in specifically is the impact that climate change might be having on severe turbulence. We can expect a 59% in light turbulence, 94% increase in moderate turbulence, and 140% increase in severe turbulence and that, of course, means we’re looking at twice or maybe up to three times as much severe turbulence as there has been historically.
Tom - So you gave us an example of severe turbulence saying this can injure people, but most of us will have only experienced, I imagine, light or moderate turbulence - the shaking feeling when you flight starts bobbing around a bit. What do you mean, is there a turbulence scale?
Paul - There is. Scientists have developed a scale much in the same way that we have the Richter scale for measuring the strength of earthquakes. We do have a scale for measuring the strength of turbulence; it doesn’t have a name but it’s a 7 point scale in which 1 means light turbulence, 3 means moderate, 5 means severe, and 7 means extreme.
So just to put some definitions there; in light turbulence which anyone virtually whose flown will have experienced, there’s just a slight strain against the seat belts. Certainly food service would be able to continue and people would be able to walk around the cabin without difficulty in light turbulence.
Let’s turn the notch up to moderate turbulence. Now there’s a definite strain against seat belts, unsecured objects certainly being dislodged, walking difficult, flight attendants instructed to take their seats.
Now let's move up to severe turbulence; this by definition is stronger than gravity. So passengers would be forced violently against their seat belts, food service and walking are certainly impossible, and because it’s stronger than gravity any unbuckled passengers and crew will be potentially catapulted around inside the cabin. So it’s this severe turbulence that really hospitalises people and it’s the kind of turbulence that’s not just an issue about comfort but about safety.
Tom - Okay. We’ve talked a lot about turbulence and how, as you’ve said, it’s going to increase in the atmosphere because of climate change. So how is that going to happen, how does turbulence form?
Paul - Yes, that’s the million dollar question! What it all boils down to is something that meteorologists refer to as a wind shear. That’s a complicated term but it means something very simple, which is simply the fact that the higher up you go in the atmosphere the windier it gets. So, for example, anyone who’s ever climbed up the Eiffel Tower will know that when you’re at ground level it’s usually not very windy, and by the time you’re about halfway up usually it’s getting quite windy by that stage. But, certainly, by the time you reach the top of the Eiffel Tower, usually the wind is blowing very strongly and that’s a wind shear. In fact, we know that this effect, the increase of wind speed with altitude, takes place from ground level not only up to the top of the Eiffel Tower, but beyond. All the way up to many kilometers; maybe ten kilometers high to form the jet stream. It’s the instabilities within those wind shears that generate turbulence.
In simple term, what climate change is doing is that it’s not warming the atmosphere uniformly. The different parts of the atmosphere are warming by different amounts. And specifically at 30 to 40 thousand feet where planes tend to have their cruising altitudes, the low latitude tropical regions are warming more than the high latitude arctic regions. So the north/south temperature difference across the Atlantic Ocean is becoming stronger because of climate change. It’s that temperature difference that drives the jet stream and as it becomes stronger, the jet stream is becoming stronger, the wind shears are becoming stronger and that’s the physical mechanism by which climate change is driving stronger and more frequent turbulence
Tom - And just with everything we’ve discussed, it almost seems to me like this could be the first real life experience of climate change affecting people now?
Paul - Yes. For some people, maybe focussed in the developed world, this might be one of the most obvious early signs of climate change. Of course that’s not true for people in the developing world where the impacts are much more serious in many ways about heat stress, and crop failure, and flooding etc., and sea level rise. But for some people in the developed world, especially frequent flyers, who of course make a contribution to climate change through aviation emissions, this effect might be one of the early signs of climate change. And, of course, for some people it might even be the thing that pushes them into actually caring about climate change in the first place, and maybe taking actions to minimise their carbon footprint.
11:07 - Mythconception: One Hit of Heroin and you're Hooked
Mythconception: One Hit of Heroin and you're Hooked
with Liam Farrell
This week’s “mythconception” comes from Moira, who has been wondering whether it’s really true that it takes only one hit of heroin to become hooked. Chris spoke to someone with first hand experience…
Liam - My name is Liam Farrell and I’m a morphine addict. Morphine is almost the same thing as heroin; heroin being diamorphine which breaks down into morphine in the body. But, I’m also nine years clean. I’m not answering this question as an expert; just as an addict still in recovery, so I do have some perspective.
Essentially this myth is completely untrue. In medicine, heroin and other similar strong opiates are used regularly for pain relief, for which they are uniquely effective, and addiction is almost unknown. Even longer term use, as in cancer pain, doesn’t necessarily lead to addiction although addiction to prescribed opiates has become a huge problem in the USA. Fifty Americans die every day because of prescription opiate overdose and it’s now a more likely cause of death for middle aged patients than road traffic accidents. So heroin has led many users to dependence and addiction.
Although it’s unlikely that you’ll develop a full blown addiction to heroin the first time you use it, that euphoric introductory experience can be the start of a compulsive cycle that leads to addiction. My memories are of a warm tidal wave washing all through my body leaving a really pleasant languor afterwards. Heroin acts by activating the specialised opiate receptors in the brain triggering a release of dopamine, a neurotransmitter that generates the feelings of pleasure.
Unfortunately, the more frequently you use the drug, the more rapidly your brain adjusts to the chemical changes that it causes and there are estimates that nearly one quarter of people who try heroin will become addicted. I used once every six months for a few years with no problems, no withdrawal reaction, no cravings before starting to take it more often. When I did start taking it a few times each week, I developed a physical addiction within a few weeks and after that, not getting the drug leads to a horrible withdrawal reaction which, after all these years, my memories of withdrawal still give me a chill.
So certainly physical addiction takes a period of regular use to develop. Though it’s possible that the seeds are sewn from that first hit, when you actually cross that line of actually sticking a needle into yourself. To put it simply, if you never try heroin in the first place, you don't have to worry about coming addicted to it.

14:29 - The Nerve Cells that Control Obesity
The Nerve Cells that Control Obesity
with Dr Goncarlo Bernardes, University of Cambridge
The human population is growing, and not just in terms of numbers. The World Health Organisation estimate that nearly half of all adults are now overweight. So the discovery this week of a network of nerve fibres that seem to control when fat cells store energy and get fat is potentially huge news. Biochemist Goncalo Bernardes, who's based at Cambridge University, has engineered a toxin to remove these nerve cells. Mice given the toxin all became grossly obese, despite eating a normal diet, and this suggests that manipulating these fat nerves might be a way to control weight as he explained to Chris Smith...
Goncalo - We first observed that nerve cells are also present in the fat tissue and this led to the question what happens if we can remove these nerve cells from the fat tissue , how would that influence obesity?
Chris - Right, but how can you remove nerve cells just from fat? Because nerve cells are present in the skin, and they’re also present in the brain, they’re everywhere, so how can you be selective?
Goncalo - To do so, what we have done is to engineer at toxin in such a way that this toxin would not cross the blood/brain barrier and therefore would spare the nerve cells in the brain and selectively influence the nerve cells present in the fat tissue.
Chris - And this is in mice?
Goncalo - And this is in mice.
Chris - So these are otherwise healthy mice and you’ve got a toxin now which selects for the nerve cells which are in the fat cells, so you can remove those nerve cells and see how the fat in the animal changes?
Goncalo - Exactly. Independently of the food intake of these animals. So we gave our engineered toxin to a group of animals and we followed all these mice that got fat or not. So what we have observed in the case of the engineered toxin is that the removal of the neurons from the fat tissue led to very obese mice independently of the amount of food intake.
Chris - So compared to normal animals eating the same diet, these mice that now lacked the nerve cells in their fat tissue gained prodigious amounts of weight?
Goncalo - Yes, that was exactly what we found, without having any brain damage.
Chris - Do you understand why the mice had this preponderance to gain weight?
Goncalo - So this study really points towards the reaction that there is a crosstalk between the nerve cells in the fat tissue and how this is related to obesity. We don’t know all the mechanistic aspects behind it, but that’s something we consider very exciting for sure.
Chris - Do you think this could lead us to a clue as to how we can help people or animals not to become obese?
Goncalo - We are using these findings to do exactly the reverse, and the reverse would be how we can stimulate nerve cells in the fat so that these mice, independently of their food intake, would not get obese.
Chris - Are they relatively easy to stimulate, these nerves? Is there a drug that you could administer that mimics the signals from the nerve cells into the fat cells to make this happen?
Goncalo - Yes. There are drugs that are being used that can stimulate nerve cells and these are the drugs that we are currently designing in a way that they can specifically stimulate the nerve cells in the fat, whilst sparing the nerve cells in the brain.
Chris - Do you think that in humans the same thing might be happening, or at least there might be a contribution from the nervous system leading to people gaining too much weight?
Goncalo - I think you phrase it very well in a way that when we go from smaller organisms to higher organisms such as humans, there is always a certain degree of complexity that is involved. What we are convinced is that there is a contribution from the nerve cells present in fat we can use to try to modulate in humans.

18:41 - How do things become Popular?
How do things become Popular?
with Professor Jonah Berger, University of Pennsylvania
Do you remember last year’s big craze the Mannequin Challenge? What about the Harlem Shake? Or maybe the ice-bucket challenge? Now the chances are you probably recall at least one of them, and that’s because they all went viral. Tom Crawford decided to find out if there’s any science behind why things become popular like this, so and he spoke to Jonah Berger, who’s Professor of Marketing at the University of Pennsylvania and also the author of the book ‘Contagious’...
Jonah - We see things catch on all the time. You might think it’s random, or it’s luck, or it’s chance, but it’s really a science there. It’s driven, in part, by word of mouth, what people share with one another, but also merely seeing others do something can lead us to do it and lead that thing to catch on. It’s not necessarily the best things that went out, one of the cheapest ones, or ones that have the biggest advertising, but it’s really the ones that fit with us. Our underlying psychology, why we share things, why we imitate others and those underlying STEPPS. And STEPPS is a framework that stands for Social currency, Triggers, Emotion, Public, Practical value, and Stories. Each of those is a reason that drives people to share or why we imitate others and leads all sorts of things to catch on.
Tom - You mentioned there steps and these six factors. So let’s talk a little bit more about each of them.
Jonah - The first part of the steps framework is the idea of social currency. The simple point there is the better something makes us look, the more likely we are to share it with others. So for example, there’s a great restaurant in New York City called Crif Dogs - it’s a hotdog restaurant. But once you finish eating your wonderful hotdog, in the corner of the room is a phone booth and if you walk inside there’s a rotary dial phone on the inside. If you stick your finger in one of the numbers and go around in a circle and hold the receiver up to your ear, the phone will actually ring and they will ask you if you have a reservation. Essentially hidden inside this hotdog restaurant is a bar that you walk into, through a phone booth. Never advertised, but they’re hugely successful. And if you think about why, the simple reason is that it’s a secret. And the first thing we do when someone tells us a secret is we tell someone else, right? It makes us look really good to know things that make us look like insiders, make us look like smart, special, in the know.
If you look online, for example, most things people share are positive: “Look at me I’m on a vacation”, “look at me I met a celebrity”, “look at me I’ve got a new car”. We’re not really sharing “hey look at me, I’m at the office working on an Excel spreadsheet, check out column C”. We share things that make us look smart, they make us look cool, they make us look interesting, they make us look like foodies or into sports. So social currency is all about the better something makes us look, the more likely we are to pass it on.
Tom - What about the other aspects of STEPPS?
Jonah - So another idea is Public. The idea is that the easier something is to see, the easier it is to imitate. So we’ve been talking about word of mouth and the idea that sometimes people share things with others. But sometimes the mere fact that someone else is doing something makes us more likely to do it. We may see someone wearing a certain shirt, for example, or a certain car and be more likely to do the same.
We’ve all heard that phrase “monkey see, monkey do.” The notion that monkeys do what other monkeys are doing and that makes a lot of sense, but the “see” part is just as important as the “do” part. If one monkey can’t see what another monkey is doing they can’t imitate it. If we’ve been to a foreign city, for example,and we’re trying to figure out where to go out to eat, we often use a time tested trick and that is we’ll look for a place that’s full. We assume it it’s full it must be pretty good, right? We’re using others as a signal of information. They don’t need to tell us it’s good, the mere fact that the restaurant's full, you use that as a social proof as information that must be good. But notice we can only do that if we can see inside the restaurant. If we can’t see it we can’t imitate it.
Tom - Is that almost an evolutionary trait in humans to follow the crowd?
Jonah - Think about how difficult it would be if couldn’t use others as a source of information. If every time we made a decision we had to do it entirely independently of everyone else. If you moved to a new city and you had to find a car mechanic, and you had to go to mechanic to mechanic to mechanic and ask them how good they were, ask them how much it would cost, even give them a trial run to see if it was any good - life would be impossible. So other people are a simple shortcut that often make choice faster and easier. So certainly, there’s an evolutionary advantage. It makes life better and easier to rely on others than simply relying on ourselves.
Tom - What about the thought that sometimes people want to be unique and want to be original and differ from the crowd?
Jonah - A great question. And, actually, my most recent book “Invisible Influence” talks exactly about this tension. On the one hand we want to be similar to others . With others there’s a signal that something is good and want to be a good member of the group and want to fit in. If we’re out to dinner for example, and we want to order dessert but no-one else wants to order dessert, we’ll probably skip it, mainly because we think people will look at us funny if we’re the only person at the table ordering dessert. So lots of times we go along with the crowd and jump on the bandwagon.
At the same time we also don’t want to be exactly the same as everybody else. We’ve all had that feeling where we wear a similar shirt to someone else at a party or event and we go “oh god, we’re dressed exactly the same” and we don’t like it. We don’t want to be exactly the same and so we do, as you noted, have a drive for differentiation, a drive to be unique, to stand out.
So these two things seem like they’re opposing - the drive to be similar and fit in and the drive to be different and stand out. But often we choose in ways that allow us to do both at the same time or be what’s called “optimally distinct.” We buy the same car but a different colour allowing us to signal that desired identity to be part of a group to signal something we want to communicate, but also to feel different. So we can point out a way in which we're unique and separate from everybody else.
Tom - Just finally then. If somebody is trying to make something popular, what’s your top tip?
Jonah - I think, again, when we want to make something popular we think it’s just luck. I think it’s all about getting lightening in a bottle or something along those lines. But it’s not. There’s really a science there. I would say that one tip is understand why people do what they do. Don’t think about the technology, to hop on social media and think that’s the key. The key is really the psychology. Why do people talk and share in the first place? If we understand that, if we understand how social influence works, then we can get anything to catch on. We can craft more contagious content, we can build more successful products and ideas, and we can get our stuff to become more popular.
25:11 - What is Development?
What is Development?
with Katherine Brown, Development Journal
Chris spoke to Katherine Brown who’s the executive editor of the journal ‘Development’…
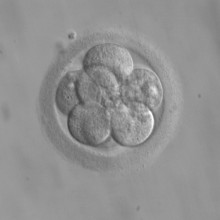
Katherine - Developmental biology, or embryology as we used to call it, has been going on for well over a century and the journal that I work with has been going for more than 60 years. Really, what we’re interest in is how do you go from that single fertilised egg, or that small group of embryonic cells, to a fully grown individual? How do the cells know what to turn into; whether they should become lung or heart or brain? How do they get to form the right shape so that your gut is a long tube, and your lungs are well branched so that you can get lots of air? And also, how do you do that with all of the organs at the same time so that everything becomes the right size at the right time and both your legs are the same length?
Chris - That’s important. But why is it important for scientists to understand that process?
Katherine - What’s really important now, I think, is that because what we’ve some understanding of this we can learn what goes wrong in diseases. So lots of developmental diseases, for example, that affect newborn babies. And we can also start to take either those embryonic cells out of an organism and into a dish and turn them into the right kinds of cells to be able to use, for example, cell therapies in various diseases, or to be be able to try and look and understand what goes wrong in a disease so we can try and fix it.
27:17 - Repairing Brains to treat Parkinson's Disease
Repairing Brains to treat Parkinson's Disease
with Professor Roger Barker, University of Cambridge
We can use our understanding of how organs form in a foetus to help to repair damaged tissues in people with diseases. To see how this is happening, Chris went to meet neurologist Roger Barker at the Cambridge Centre for Brain Repair, where he’s using human embryonic cells to developing a cell-based therapy for people with the movement disorder Parkinson’s Disease...
Roger - Cells for repairing brains can come from many different sources. And, actually, it’s one of the problems in the world at the moment is there are many clinics out there advertising cell based therapies for brain diseases, amongst others, where the origin of the cells is a little obscure and the science underpinning it is even more obscure.
In our particular case, there are two main sources I would say for cells to repair the brain. One comes from spare embryos from IVF programmes so when the eggs fertilise, the egg obviously then divides, and when it’s only a few cells old forming an embryo some of those embryos are then stored and you can turn those embryos into embryonic stem cell lines Obviously the advantage of that is that those cells, which ultimately give rise to a person, have the capacity to turn into any cell in the body.
Chris - That was where I was going to ask you the question because an embryo could be any cell in the body - an embryonic stem cell, but you want a certain kind of brain cell.
Roger - You're absolutely right. In our particular case we want to make them into nerve cells so we have to learn the instructions to give that embryonic cell to turn into a nerve cell of the brain. That is something which has evolved over the last 20 years, I would say, as people have understood more specifically how the brain normally develops and, therefore, how you can instruct these cells to follow normal development.
Typically what people do is they put chemical factors onto the cells. They put in these things called transcription factors, factors which are used by nature to activate a whole series of genes to produce particular products to push cells in directions that you want them to do. So we have a best guess at what nature’s done already to direct our cells down normal development to produce the same cells which we want to them transplant ultimately into patients.
Chris - You said there are two approaches - embryonic stem cells being one. What’s the other?
Roger - The other one is a newer technique which has come about in the last 10 years from pioneering studies in Japan, and these are called Induced Pluripotent Stem cells. The principle here is that you can take an adult cell, so classically you take a few skin cells, and then you can turn them back into something that looks like an embryonic stem cell. So you reprogramme it back to its very origin. Those so called IPS cells have the same potential as embryonic stem cells but they have less ethical baggage attached to them because of their origin. But they are newer so the science is a little bit lagging behind where we are with embryonic stem cells.
Chris - Is that why you’re going down the embryonic stem cell line route at the moment?
Roger - That is the reason we’re choosing embryonic stem cells because they’ve been around for longer and our techniques work more robustly with those. Ultimately, IPS cells may be the preferred option and one of the areas people are particularly attracted in is this so called idea of using the patient’s own cells to repair their own brain.
Now that is a very attractive idea but there are a number of problems with it. The first is the cost because in order to make a personalised treatment for you it would probably cost somewhere in the region of 2 or 3 million pounds currently. The other key problem, of course, is if you develop Parkinson’s disease in the first place so now I’m going to make some dopamine cells from your own cells, your own genetic background, which gave you Parkinson’s disease in the first place. So there’s a worry that you would now develop Parkinson’s disease in the same cells that I’ve put in to treat you for your Parkinson’s disease.
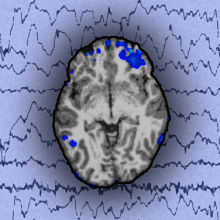
Chris - Which means your rationale for using embryonic stem cells may be a sound one. Is that what’s in the dish on the microscope here?
Roger - Well, what we’ve actually got here is embryonic stem cells that have been partially differentiated. So then this particular image we can see here, they’re not embryonic stem cells, they are cells which have now been moved into a neural precursor cell state.
Chris - So, essentially, are you fooling it into thinking it’s in a developing embryo in the place where the brain would be surrounded by other brain cells, but you’re simulating that chemical melia and the cells are fooled into thinking they have to turn into something like that?
Roger - That’s exactly so. So you’re trying to convince this cell that it wants to turn into a brain cell. The next trick you’re going to have to do is say well, I don’t want you to just turn into any old brain cell, I want you to turn into a particular brain cell which, in our case, we want to turn them into dopamine cells.
Chris - You can do that?
Roger - We can do that and we think we can generate reliably with this technique large numbers of dopamine cells of the type lost in Parkinson’s disease.
Chris - And when you say large numbers, in a patient who has Parkinson’s disease and you want to treat them, how many cells are you going to need?
Roger - Probably not many. In Parkinson’s disease, you actually lose a quarter of a million dopamine cells on each side of your brain; that’s what gives you the Parkinson’s disease. So, in theory, if you can replace a quarter of a million, which sounds like a lot in the context of 80 to 100 billion which is what you have in your brain, it’s not a lot.
Chris - How do you get a cell into the brain?
Roger - That’s pretty easy. Essentially, in order to transplant them in, what we do is concentrate the cells into a little vial. They’re then taken up into a specialised needle and then in neurosurgery them make a little hole through the skull, they locate where they need to implant the cells and then they essentially put the needle in and put little droplets of cells along various tracts where we need it.
Chris - What do the cells then do after you’ve put them in?
Roger - Once the cells are implanted, some of them will obviously die, but what the majority will do is that they will sit there and they will slowly turn into what we want them to turn into, which is the dopamine cells. So they will take on the characteristics of the dopamine cell, they’ll put out a process which then makes contact with the patient’s brain, the brain’s own cells will make contact with it, and then it will slowly mature.
One of the interesting aspects, if you like, is it will take a long time to see the maximum effects because this cell has to bed down, differentiate into it’s final type of cell, and then integrate and mature. In the case when we’ve used not embryonic stem cells, but we’ve used foetal dopamine cells, it can take 2, 3, 5 years for those transplants to have the maximum effect.
Chris - How do you know they’re surviving because, obviously, you can’t go back in that person’s brain when they’re alive and see what the cells are doing so how do you follow them up? How do you know that what you’ve just said is what is happening?
Roger - Well, you could take a very simple approach and say Parkinson's’ disease is a progressive disease; people get worse as the years go by. So if they got better, and continued to get better, the implication would be that the cells were doing what they were supposed to do. Now we are also very fortunate in the case of Parkinson’s disease because we have scans now that can look at chemicals in the brain and these cells are, obviously, producing a thing called dopamine, and we look at dopamine in the living brain. If our transplant survives and has the effect we expect then the dopamine levels will stabilise and then improve as your transplant matures.
Chris - And finally Roger, in terms of the effectiveness of this therapy, if you’ve got someone who’s very disabled by Parkinson’s, what sort of scale of difference does it make to their daily life if they were to go through this process?
Roger - These therapies have the potential, not to cure Parkinson’s disease, but to dramatically change the natural history of treating Parkinson’s disease. So, if they work as well as we think, then in essence they should get rid of all of their need for medical therapy. In fact, when they work well, they transform people’s lives pretty much back to normal.
Chris - Exciting, isn’t it? That was Roger Barker, he’s at the Cambridge Centre for Brain Repair. Still with me is Katherine Brown the Executive Editor of the journal Development. Katherine, what’s your reaction when you hear this sort of thing?
Katherine - I think Roger’s work is really exciting, and the reason I think it’s so promising is the way that he’s done it. In really trying to understand how the body made those cells in the first place so that when you try it in a dish we’re recapitulating that process and we can really make sure that we’re making the right cells to put into the patient so, hopefully, they’ll have the right effect.
Chris - Are there not risks with embryonic stem cells; could you not end up with a cell that isn’t a brain cell in your brain and that could be bad for you?
Katherine - I think it’s always a risk and that’s something that people are being very concerned about. The embryonic cells, they’re young by nature and Parkinson’s is a disease that tends to affect older people. So one of the things that’s a real challenge in the field, not just with Parkinson’s but with other diseases, is to try and make those cells more mature and more like an old person’s cells so that we can use those to treat these diseases. We’re making progress on that, but it’s not completely there in all cases.
Chris - One of the other things Roger alluded to was that you’re making cells and putting them into a diseased environment. Something in that brain gave that person Parkinson’s disease to start with so there’s an additional problem to surmount which is we’re putting cells back into a non-ideal environment potentially?
Katherine - Absolutely. And that, again, is why some diseases are more likely to be amenable to these treatments than others. So in the case of Parkinson’s, we know quite a lot about the genetics of the disease and we can, hopefully, be able to trigger this and we’ve got some evidence that it works already, but there are only some diseases this will work for.

36:21 - Organoids: Miniature Organs
Organoids: Miniature Organs
with Dr Hans Clevers, Ubrecht Institute
Adult tissues contain stem cells, which means that, under the right conditions, they can be cultured and turned into new miniature organs - called organoids - to study how diseases happen and what drugs might best benefit an individual patient. One of the pioneers of this approach is Hans Clevers from the Ubrecht Institute in the Netherlands who spoke to Chris Smith...
Hans - Organoid is a very small artificial version of a real organ grown in a dish, typically from stem cells. These organoids represent many aspects of the original organ from which they were taken.
Chris - Why would you make one?
Hans - There are various reasons to make organoids. One would be that you’d like to study an organ in great detail, which can be done in animals. It can be done in people but can be very difficult. It’s much easier if you take some cells out of a human being and culture them in the form of a mini organ and study them with great care.
Chris - Now you make that sound very easy - I’m sure it’s not. So what steps do you have to go through in order to take a bit of my tissue and make a mini intestine of mine, for example?
Hans - The best example would probably be a mini gut that we could make up of your colon or you small intestine. We learned about ten years ago what the stem cells are and what they look like in the inner lining of your gut. Stem cells are the cells that will repair or replace lost cells that wear out or when you’re sick you lose them. Stem cells become active and they divide and replace the lost cells.
So we’ve learnt where they are. We then learnt how to keep them active, how to mimic the situation that occurs in your gut. Mimicked in a petri dish, which means that we grow them 3D in a gel and we have to give them growth factor. What we grow is the lining of the intestine so this is the tissue that helps you digest food and take up the nutrients and pump it into your blood and lymph vessels. We do not produce the muscle that surrounds the lining and helps to move the food through your intestinal tract, and also we don’t have blood or lymph vessels. So, in that sense, they are somewhat incomplete.
Chris - But nonetheless, because there are lots of diseases that affect the lining of the intestine, being able to produce this in a dish means that you could then model what a disease does or how it works in a dish rather than having to go to the patient?
Hans - Yeah. We call the lining the business end of the gut; that’s where all the gut stuff happens. Many diseases are, indeed, associated with a complex set of functions that’s located there and often diseases are associated with a loss or changes of these functions.
Chris - What sorts of things, once you’ve taken a sample of these stem cells and made an artificial mini gut lining in a dish, what sorts of things can you test on it?
Hans - Probably the best example I could give is an application where we use this to advise doctors who treat cystic fibrosis patients. Cystic fibrosis is a simple disease and always affects the same gene. The problem is there are lots of different flavours of this disease and only for the more common flavours is there a drug. These drugs are extremely expensive, up to half a million US dollars per year per patient, and it’s very unpredictable for who they will or will not work.
What we found out is that you can actually test whether a drug works in a patient that was known but it’s very expensive if you have to treat a patient for about a year to figure out if it’s okay or not for that particular patient. But we could create an avatar of that patient (a mini gut). So we take a small rectal biopsy; it’s a painless procedure; grow it for one or two weeks in petri dish and get maybe 50 or 100 small mini guts. Expose these mini guts to the drugs and there is a one to one, black and white correlation between restoring the response with the drug in the petri dish and then giving it to a patient and see the patient respond.
Chris - Can you pull off the same trick with pretty much any organ in the body or are you confined to just studying the guts?
Hans - The gut was the first one that we tamed in the lab but, since then, we have come up with protocols for almost any internal organs, so lungs, liver, stomach, prostate, ovary. In all cases, if we play around with the conditions a little bit, we can come up with conditions where the stem cells from that particular organ will make a mini organ in culture. The exceptions, we think, are going to be heart where we fear there is no real stem cell, and brain where there is very little, if any, stem cell activity.
Chris - Notwithstanding that, that means you can then produce miniature versions of those organs to do things like test drugs, or test disease processes, and give people predictions about the diseases?
Hans - Exactly. And one other major field that we can actually do this with normal tissue, which was essentially impossible before we developed this technology but we can do it more easily with the cancers that develop in those tissues. And there again, the promise is (and we are currently testing this) that one could grow a cancer, for instance of the colon, of the pancreas, side by side with the normal tissue from that same patient. Expose the mini organs and the mini cancers to a variety of cancer drugs and then score, much like we do with cystic fibrosis, for the best cancer drug for that individual patient, knowing that overall probably a third of the patients benefit from their first chemotherapeutic treatment but all of them will get the side effects.
Chris - So we might be able to come up with much kinder chemotherapy regimes in the future, thanks to that work. That was Hans Clevers from Rotterdam.
Chris - With me Katherine Brown, executrive editor of the journal development. Those things are being used to understand processes, and diseases, and drug treatments, but they’re not actually being used therapeutically are they?
Katherine - No not yet, and I think we’re quite a long way from that. People have shown, in fact, in a mouse, taking Hans’s mini gut as an example that if you injure a mouse intestine and you put one his mini guts into that intestine, it will engraft and it will restore some functions. But that’s in a mouse, and that’s very early work but that is some promise that we may be able to use that in a subset of cases to treat certain diseases.
Chris - But what is the key difference between an adult stem cell and the embryonic stem cells that Roger was talking about. If adults have stem cells, why do we need to every use embryos?
Katherine - There are various differences. I think the most important thing is that not all of our tissues have large populations of stem cells and we don’t necessarily understand them very well. As Hans said, the heart for example, as far as we can tell the adult human heart doesn’t have stem cells in it.
The other big difference I think that is really important is that adult stem cells can only become certain kinds of cells, so they’re much more restricted in what you can do with them. Whereas an embryonic cell, because in normal life it has to make everything, when you put it into a dish it can also make everything.

43:35 - Organs on Chips
Organs on Chips
with Dr Don Ingber, Harvard University
By understanding development scientists can repair defective organs and grow miniature versions of the organs, but they can also go one step further and create an entire working network of the human body in the lab. This is what Harvard’s Don Ingber is doing as he explains to Chris Smith...
Don - Organs on chips are sort of a minimal model of a human organ. I think what’s novel about what we do is that we’re able to create tissue-tissue interfaces which is what defines an organ. Usually there’s a blood vessel involved as well as the specific cell types, of the lung, or the gut, or the kidney. We use computer microchip manufacturing methods, and that’s why we call them “on chips” to create devices with hollow channels less than a pencil lead wide (less than a mm wide), that have two channels with a membrane between.
We are able to culture the cells from the tissue, and the cells from the blood vessel on opposite sides and we can perfuse it with life sustaining medium. If it’s the lung we could put air on top of the lung cells and we could recreate the physical environment - breathing motions in the lung, peristaltic like motions in the gut, which we find are absolutely critical for function. We do this either with cells from normal people, or from patients with diseases, or with IPS cells as you heard about earlier (Induced Pluripotent Stem cells), which we can induce to form specific organ types.
I think what’s unique about our models is because we have a blood vessel, we could really begin to study how drugs are distributed to and from each organ and we could link multiple organs by a common blood vessel channel to create what’s effectively a human body on chips. We have created over ten different organ chips so we really have a pretty good representation at this point.
Chris - So it’s a bit like what Hans is doing with his organoids except you’re making a smaller representation and, rather than an isolated miniature gut, you’ve actually got an isolated miniature gut, and a liver, and other organs and they can share a blood flow, if you like, which means you can then understand how these things would interact chemically and biochemically in the body?
Don - That’s right but there’s more to it in that what’s different is we’re inspired by nature, we’re not trying to rebuild it precisely. We put the cells in the right environment, give it the right physical environment, and let them undergo their own developmental process in our chips, just like they did in the embryo. So, for example, when we put the lining of your gut epithelial cells on our chips and we give them flow, and peristalsis like motions, they spontaneously form the villi (the fingerlike projections that you see in your gut), and you put the cells of your small airways, they spontaneously develop cilia and mucus and move the mucus at normal rates. It’s really quite amazing to see that the cells are programmed to undergo their own developmental protocol, if you give them the right physical microenvironment.
The other thing about the blood vessel is that we could put circulating immune cells through these chips and, therefore, study inflammation which is a major part of most diseases, if not all, and that’s something that’s very hard to do in organoids or any of these other systems.
Chris - So would you have then a dish which has got a miniature liver growing in it, and you would have a channel connecting that to all of the other organs that you’re modeling in their dishes, so they share a common blood supply, if you like?
Don - We don’t have any dishes - we have chips. The chips, as I said, have these little hollow channels. Each ones the size of a computer memory stick; they’re optically clear, made out of a flexible polymer so we can stretch it so that it can breath, like I was saying. But you have very high resolution, real time imaging, so you basically have a window on molecular scale behaviours inside cells, inside tissues in an organ level context.
We then transfer the liquid from one blood vessel channel chip to another. So, for example, we might have a drug that we put through the lumen of the gut chip, take the outflow of the blood vessel channel and move it to a liver chip, and a kidney chip, a bone marrow chip, and a blood-brain barrier chip, and a heart chip, and so on and so forth. We can transfer back and forth and we can actually, using computational modeling, we can begin to predict how drugs are distributed throughout the body, how they’re cleared, metabolised, and what’s known in the pharmaceutical industry as pharmacokinetics, pharmacodynamics, which is you want to understand how to dose a drug, what concentration you should give to get it where you want. Then pharmacodynamics is the efficacy and potency of the drug with the goal being you might be able to test drugs on these chips and predict, extrapolate if you like, the results from the chips into what goes to start in a clinical trial in humans.
Chris - I suppose, at the moment, a lot of that work is being done in animals, which are not necessarily the best model in all cases for certain drugs to test and, therefore, if you have a more relevant way of modeling how these chemicals might behave, then that’s got to be a good thing?
Don - It’s exactly right. There are many examples where there are drugs that never had toxicity in animals and they went to the clinic and they had major toxicity and were pulled out. For example, we are able to mimic pulmonary thrombosis on chips and we see this with drugs that failed in clinical trials for that reason where they never saw it in animals.
We’re also able to mimic very complex diseases. We make chips with cells from human patients with COPD (chronic obstructive pulmonary disease), who are known to be very sensitive to exacerbation of disease by cigarette smoke. Then we developed a cigarette smoking robot, and it literally takes real cigarettes and puffs them and the smoke goes into the chip, and we recapitulate the phenotype seen in patients. They are much more sensitive to cigarette smoke in terms of inflammation and injury compared to normal chips.
Chris - Can you then look at knock on effects beyond just the lung because that’s the other interesting thing about smoking, is that it doesn’t just affect your lungs where the smoke goes. Every organ sees the chemical byproducts of smoking and, therefore, there are disease consequences for everything so can you see that?
Don - We haven’t looked at that yet but we can measure metabolites… we can easily do that by linking the chips together. But we have certainly looked at the effects of drugs on multiple linked organs. We’ve done eight so far linked with one drug and, actually, it was nicotine which you get with cigarette smoke so, in a sense, we have looked at that. I hadn’t even thought about it that way but we’re looking at nicotine which is one of the things that is absorbed and does affect the functionality of other organs. But that is the idea.
The other part of this is that you can imagine taking IPS cells that you heard earlier (the adult stem cells) from a group of patients who are genetically similar, and then developing a drug for that genetic subgroup. Then using that group for a small clinical trial which could greatly shortcut the whole process of drug development where now they usually do large groups, fail, have to search for a subgroup and, if they’re lucky, find it and do a small trial and then get approved. So this can really turn drug development upside down.
Chris - Still with me Katherine Brown from the journal Development. Katherine; what are your reactions to that?
Katherine - I think, again, this is really future looking work and what’s particularly exciting is that we can actually start to look at things in a systematic way. Look at multiple organs together and try and figure out what happens when you take a drug, which is probably intended to act on one particular cell, what’s happening to all those other cells, and whether there might be negative consequences.
The other thing I think is really exciting is that we can do this with human cells. Because so much of what we’ve done in the past, both from developmental biology and also when we’re looking at disease, is use animals as a proxy for this. And being able to look at humans from both of those perspectives, I think, is now really important and should really drive those fields forward.
Chris - The one thing that Don didn’t mention is whether or not he can model the microbiome because we’ve already heard in this programme we’ve heard a slew of new stories in recent weeks to years about the role that the microbes that live in us and on us play in helping the body to develop. Things like the blood-brain barrier and so on with this story about penicillin in pregnancy. I suppose one ought to consider also that there’s a lot more to development than just growing cells in a dish. There’s a three dimensional environment; there’s also a chemical environment and outside influences like Don was saying with stretching things, and so on.
Katherine - Absolutely, and that’s something that’s really important and really up and coming in the developmental biology field at the moment is our understanding that if stretch, or compress, or poke a tissue, that can actually change what happens to those cells. Similarly, those bacteria, we know they’re sitting on your gut lining, we know they're doing important things there, and we are actually beginning to understand they’re not just doing important things in the adult, but they’re doing important things in development in order to make your tissues what you need them to be.
Chris - Now I’m going to put you horribly on the spot now. Just with your horizon scanning, editorial hat on, what do you think are going to be the really big things up and coming in your field either this year, or in the next couple of years or so?
Katherine - As I’ve already said, I think the fact that we can use human cells to try and understand this is really exciting. I’m a developmental biologist and so one of the things I’m really interested in is what is it that makes humans different from other animals? One of the things that people are already starting to learn about, but we’re going to get much more in the next year or two is, for example, why is it the human brain is so much more bigger, and so much more complex than the mouse brain? What makes it grow big, what makes it fold, and what makes us able to do all the things we can do that other animals can’t?

54:18 - Why do Bombs make a Whistle Sound as they Fall?
Why do Bombs make a Whistle Sound as they Fall?
Izzie Clarke spoke to Professor Peter Main from King’s College London to sound out George’s question…
Peter - Most of the missiles shown in documentaries and films refer to the Second World War and for the typical height of those bombers, the falling missiles are accelerating, but not sufficiently to break the sound barrier. That means that apart from a relatively gentle whoosh, they would not naturally make any sound. However, it was in the interests of the bombers to terrify those under attack so, often, an artificial whistle was incorporated into the missile.
Izzie - You hear right - they added a fake whistle. But what does that mean for missiles that travel in this hypothetical endless hole?
Peter - If the missile could fall under gravity further, accelerating all time, after falling about 5,000 metres it would reach the speed of sound and would then emit a sonic bomb, just as supersonic aircraft do when then fly at speeds greater than the speed of sound.
Izzie - This happens when objects travel faster than 343 metres per second. The air molecules are pushed aside with such a great force that it forms a shock wave. It sounds a bit like a thunderclap. So how does the missile sound relative to the pilot?
Peter - In principle, if the pilot of the plane could have heard the whistle, he would have heard it in the way described - a high pitched sound, falling in frequency according to the well known Doppler effect. This is the same effect as when say a police siren changes pitch as it approaches and then passes by, and is due to the motion of the object compressing the wavelength of the sound as it approaches the observer - that is increasing its pitch and stretching it as it moves away.
Izzie - Someone on the ground would actually hear the pitch increase. In other words, it sounds higher and higher as it approaches. So that means those beloved filmmakers are using the wrong sound…
Peter - Well, that’s because the sound has nothing to do with bombs or missiles; it’s a special effect created in the studio. The particular sound with the frequency of the whistle falling has become a cinematic convention, which explains its common use in many films.
Related Content
- Previous Battle of the sexes
- Next What noise does a falling missile make?










Comments
Add a comment