Why don't microwaves spark off themselves?
The Naked Scientists tackle your questions, from how hail storms come about to why the Mediterranean Sea has such small tides. And why do people often favour walking on one particular side of the road? Plus, we look at what science might hit the headlines in 2014, from China's ambitions for manned spaceflight, to new graphene-based electronics.
In this episode

- China's latest move in the space-race
China's latest move in the space-race
China has the world's most ambitious space programme, and 2014 will see the nation take some important steps forward.
A new space launch facility at Hainan Island should be completed by the end of the year, allowing the  country to launch the new generation of Long March 5 heavy-lift rockets.
country to launch the new generation of Long March 5 heavy-lift rockets.
That's what the country needs to follow a roadmap recently laid out by the Chinese Academy of Sciences, which would include establishing a manned lunar base and launching astronauts to Mars by 2050.
For now, China's Jade Rabbit rover, which landed on the Moon in December, is busily exploring its new home. And plans are taking shape for a second orbiting space lab, Tiangong-2, in 2015.
By 2020, they may have a full space station in orbit - just in time to take over from the International Space Station.

How can I make fireworks different colours?
Mark - I suspect it's because if you're detonating gunpowder, the reaction is simply too quick. Everything is going to be blown apart before you actually get a chance to excite those metal atoms and start them emitting the colour of light that you want. Fireworks are constructed in a very particular way to actually make those metals burn. It's a slightly slower burn as the fireworks go off. That's why when you see fireworks go off, you've got strontium in there and that's making this intense red, barium for green, and so on.
Dave Ansell - The other effect is that if you've got lots and lots of carbon in there, carbon tends to glow. If it hasn't actually burned completely and really combustive carbon glows bright yellow. So, if you're not careful, that's going to completely overwhelm your nice colours in the fireworks as well.
Mark - Did she say what colours she got in the explosions?
Chris - No. She just said it didn't work very well.
Mark - Well, that is a shame.

Can light age?
Dominic - Well, light is made up of these particles we call photons which seem to basically be quite timeless. They don't age when they're travelling through free space. They can be absorbed by things they pass through. But it's actually quite useful particularly to people like me who are astronomers that light can travel for billions of years through free space completely unchanged because that means we can look back through the universe and see light that was emitted billions of years ago, and see what objects in the universe look like in the very distant past.
Dave Ansell - The one way it does change if it's come an awful long way is it can get red shifted. So, things which are an awful long way away from us seem to look redder than they should do because the space inside is stretched and they stretch the photons.
Dominic - That's right. When you're looking at these very old objects, you're seeing the fact that the universe is expanding around us, over periods of billions of years. As that universe expands, light has a particular wavelengths associated with it and that wavelengths get stretched. Red light has longer wavelengths than blue light. So, this light becomes redder as it gets older.

How much toast can a lightning bolt make?
We're nearly at the end of the show, so I should give you the answer to our toast question. It's about 100,000, as Ed suggested, because basically, there's 10 billion joules of energy in a lightning bolt and it takes about 100,000 joules to bake a slice of toast.
So, if you divide 10 billion which is 10 to the power of 10 by 10 to the 5, which is 100,000, you get 10 to the power of 5, or 100,000...
06:25 - Graphene in mass market manufacturing
Graphene in mass market manufacturing
Graphene looks set to take off in 2014, with applications in mobile devices and touch-screens. Mark Peplow explains...
Mark - One of the  things that's going to potentially be quite exciting this year is that we're actually going to start to see products that incorporate graphene on the market.
things that's going to potentially be quite exciting this year is that we're actually going to start to see products that incorporate graphene on the market.
Graphene is this 1-atom thick sheet of carbon arranged in a sort of hexagonal honeycomb pattern.
It was first isolated just 10 years ago and it's got some remarkable properties. It's stiff as diamond, hundreds of times stronger than steel weight for weight, but it's extremely flexible. Electrical charge travels through it much, much faster than other materials like silicon.
So, scientists have spent the last 20 years trying to investigate its properties and find uses for it.
There have been the old one or two very neat products on the market, but this year, we're being promised actual commercial devices. The first major one is likely to be touchscreens for Smartphones.
Chris - How does it work that's different from the silicon that we use at the moment or other similar materials of semiconductors we already have?
Mark - Well, let's look at a touchscreen. That's made out of something called indium tin oxide and it's electrode basically. So, when your finger touches it, it changes electrical current going through it and the device can sense where your finger is. That's why your phone knows where you're prodding it.
The key thing about indium tin oxide is it's transparent because you need to see the stuff going on underneath it that your phone is projecting. The trouble is, it's quite brittle and indium - the key ingredient in it - is increasingly rare, so it's getting more and more expensive.
Graphene potentially has the same properties and it's transparent and can conduct electricity very well. So, it could be a much more robust replacement. The key is going to be manufacturing it cheaply enough and at high enough quality.
Chris - When they were making graphene in the early days, they used to basically peel off bits with Cello tape from pencil leads and things. So presumably, they're not using that method to make sheets of graphene for mobile phones. They must've got this right now. How are they doing it?
Mark - That's how it was first isolated. Yeah, you literally take some graphite which is stacks of graphene, billions and billions of them all stacked like a huge sort of Shaggy and Scooby Doo sandwich. You just use sellotape to peel off the top layer. That is still the best way to give you the highest quality graphene.
Other larger scale manufacturing methods still can't make that quality. But the leading one is called chemical vapour disposition, basically use methane which is a gas and it's got carbon in it. You pip it over this copper foil. It's heated up to about a thousand degrees and that breaks down and forms these sort of sheets of graphene that slowly accumulate over it and then you very, very delicately peel it off and try and stick to whatever backing you need.
Chris - Who's making this and what are we going to see it in and when?
Mark - Well, the largest scale manufacturing operations over in China and it's remarkably difficult to actually get details about where they are with this. Samsung, the phone maker have already been showing around prototype devices that incorporate graphene touchscreens.
The big question is, can you make it good enough quality cheaply enough to make it a reasonable replacement? There's lots of industry watches saying that the prices are going to come down enough, the manufacturing is going to improve enough that it's going to happen this year. There's certainly a lot of Chinese companies that are making a lot of these stuff. So, we'll just have to wait and see.
Chris - Brilliant! Mark Peplow's prediction for 2014. Thank you very much, Mark.

Why do people walk on the left or right?
Chris - I think there are two aspects to this. I don't know what you guys think, but I mean for my fourpence, I would say first and foremost that in the same way that fish make a big shoal and they all swim in one sort of direction once someone's shown everyone where to go. I think that an element of this is that once one group of people are doing one thing, people tend to follow. So, if everyone happens to be on the left then more people are going to go on the left than on the right because we do tend to - because we're social animals - copy each other. But also, there is an intrinsic brain bias towards certain parts of the world we live in, in terms of how we attend to it and how we tend to favour it. Dominic who's very interested in computing and website design will know this very well that anyone who designs websites is told, "Put the most important content..." Where Dominic?
Dominic Ford - On the left hand side because people read from left to right. The first thing they see is on the left.
Chris - Absolutely, so your most important content at the top and on the left because that's where people visually attend to more. And also, for some reason, we think that that part of the brain that's observing that vision on the left is also more receptive to information being presented in that way.
Dominic - I think there's also a social aspect to walking on the right side of the road because hikers are actually often told to walk on that side because then you've got the traffic like on the left coming towards you. So, you can see the car that's about to come very close to you. Whereas if you walk on the left, you can't see the car coming up behind you.
Chris - Good point. Les, are you running people down? Is that what it is?
Lez - No. I deliberately go on the left just for the hell of it.
Chris - I like that. You know, if you're a racehorse then horses actually perform much better on races run in one direction than the other. They tend to have an intrinsic bias for one side over the other. The majority of horses I think are right-legged and so, they prefer to run with that leg as their leading leg. If you run them in the opposite direction, they're at something of a disadvantage. I don't know why they should have that particular bias because we think humans have a side bias because as Dominic pointed out, there's language in the brain. We think that language being on one side of our brain makes that side of the brain dominant and that might be the reason.

Why do trolley wheels rattle?
Dave - I guess, this is down to sort of how much energy absorption there is because if you're driving over a very, very hard surface which is a bit rough then if your wheel drives into even quite a small bump, then the only thing that can happen is that the wheel has to get after the way of that bump so the trolley is going to move up. Imagine that they're really quite fast because it's got to get over that bump quite quickly. And so, it will get thrown up and then the trolley move, fall back down again and go thump especially if it's very light. If there's a lot of weight on there then the tires might deflect instead. But when it's very, very light, the trolley is just going to move up and it will bounce and rattle. Whereas if you're on a soft material like grass, then there's two options. Either the trolley can go up or the ground can go down. If you're lucky then the ground will go down and so, it shouldn't throw things up and down as fast, and should be a lot quieter.
Dominic - I think you're right. It's about the absorption because the grass is softer, so it's a more absorbent material. So, that means it can absorb the energy heating up the grass whereas concrete is very hard. So, that's not going to absorb the energy by deforming. It's going to have to dissipate that energy by sound.
Chris - One thing that's quite interesting in my experience, I don't know if you've ever driven on dirt road. Either of you have driven on a dirt road? And you get corrugations forming on the road. Have you seen this? Have you seen this, Peter?
Peter - I can't say I've driven much on dirt road, but what you're basically saying is the direction of travel. It's like going across furrows, the corrugation.
Chris - Yes, that's right. You see these corrugations, you think, where do they come from and actually, this is down to the oscillations of the shock absorbers on the vehicles, because as the vehicle goes up over a little bump, the shock absorber has a damping effect. And so it takes time for the car to stop moving up and down. That oscillation is pretty standard from one vehicle to the next. So, it ends up pushing lots of dust into little ridges that slowly build-up to make these corrugations in the road. I thought - when I was driving on these in the middle of nowhere in Australia - because if you drive slowly on them, it rattles the car to pieces - I thought "they must be made of rock or stone or something." In the end, I got out and went over to one of these things and kicked it, in frustration, because it was slowing me down so much - and it's just dust. So I thought "well, if I drive really fast actually, then the period of oscillation of my shock absorbers will be out of phase with the people who've gone along here slowly. And, therefore, I will miss these bumps and therefore it should be a gentle journey because I will just be like driving over piles of sand. So, I tried it and, instead of going 50 km an hour I went 70 for a bit, and it solved the problem. So, you're driving your trolley too slowly, Peter! That's what it is! Go faster!
Peter - [laughter]. Okay, I should mention that the trolley doesn't have tyres. We're talking solid nylon wheels here. So, your explanation would actually fit very well. But it does mean that the supposedly smooth paved surface actually is far from smooth. It wasn't actually concrete. It was apparently smooth tarmac. So, even that must have sufficient grain to cause quite a lot of vibration.
Chris - Indeed. Well thank you very much, Peter. It's very kind of you to phone in. Good to have you on the programme.
Peter - Very interesting answer, thank you.
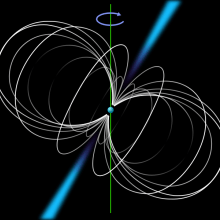
How fast does gravity propagate?
Chris Smith discussed the answer with Dominic Ford and Mark Peplow...
Dominic - So yes, gravity is this force that binds everything in the universe together. There's this quite fundamental principle in physics that nothing can travel faster than the speed of light. And so, physicists have rather suspected on the basis of that that gravity must propagate at a finite speed - at the speed of light - because if it happened instantaneously, then wobbling something from side to side in the Universe would essentially be propagating information about how that thing was moving faster than the speed of light and violating this very fundamental principle.
It's been obviously quite hard to test because trying to find some experimental setup where you test whether gravity propagates faster than the speed of light, is really quite a challenge. But actually, in the last 10 years or so, we have done that with objects called pulsars which are very compact neutron stars. They are basically the mass of a star in the size of a mile or two across. Some of these things are very close to one another and spinning around each other very fast. Actually, Einstein's theory of general relativity which is the best description of gravity we have, predicts that when these things are orbiting very fast, they should produce what's called gravitational waves which are ripples of gravity, that travel out at the speed of light. If they're doing that, they should be gradually losing energy through these gravitational waves.
In fact, we have found pulsar binary pairs that seem to be gradually getting closer and closer together as if they're losing energy, at exactly the rate that Einstein predicts, if gravity travels at the speed of light.
Chris - Mark...
Mark Peplow - As the pulsars change their rate, you're sort of inferring the existence of gravity waves. What would it take to detect the gravity waves themselves?
Dominic - Well, there are a number of teams around the world who are trying to build detectors to detect these ripples of gravity moving through space. The sensitivity you need to that is absoloutely mind boggling. You're talking about distances of about a mile that you're sending light beams down and you're trying to see whether gravity is causing that distance to ripple by about the size of an atom. So, you've got an experimental setup a mile long and you're trying to detect something that's moving by the width of an atom. No one has yet detected those gravitational waves.
They are, I think, getting quite close incredibly. I'm always quite incredulous when I hear about these experiments because they sound bonkers to me. But I think in the next decade or so, we might actually start to detect these ripples in space time...

How much was understood in ancient India?
Dominic - Certainly, there is quite a lot of interesting science in India. One of the reasons for that is to do with legacy of ancient Greece which actually travelled to India. I think it was through the Persian empire and in fact, in the first few centuries AD, India was quite a big mathematical centre because ancient Greek knowledge had been lost in the west but it was still flourishing in the east. It was only in fact in about the 12th century, it came back to the west. In terms of nuclear physics, I have not heard anything about that. I certainly heard lots about ancient Greek astronomy being found in texts in India, but I don't think the ancient Greek has ever got anywhere near nuclear physics.
Dave - Though there was a nuclear reactor, a very, very ancient reactor which developed somewhere in Africa, but this was millions of years ago when a load of bacteria obviously evolved to collect uranium which have kept them nice and warm. They collected so much that it actually went critical and you actually look in the rocks, you can see the results of a nuclear reactor a bit form a couple of million years ago. But it was anything to do with humans...
Chris - That's called a geo reactor or something, isn't it?
Dave - Yeah. Something like that.
Chris - That they're actually bugs living in the Chernobyl nuclear power station. There's a kind of fungus that's evolved to make extra large amounts of melanin, the same stuff that makes our skin black. Because it's a very electron-rich molecule, it's very good at interrupting the kinds of particles that give rise to the radiation that's coming out of the core of that melted down reactor. So, the fungus is flourishing on the inside of the reactor vessel even now. So, it shows that bugs can tolerate really quite extreme conditions, can they?

Why don't microwaves spark off themselves?
Dave - It's a very good question. So, the reason why metal sparks in a microwave is that the way a microwave cooks is it essentially applies a very rapidly changing electric field inside the box. It does that using things called microwaves which is a form of light and it causes electric currents to flow backwards and forwards in your food. If you put a piece of metal in there, it conducts much better than your food and you'll get currents flowing backwards and forwards in that metal. Now, if that metal is on its own and insulated from everything else, that will just cause a current to flow through it and it will get very, very hot and you'll have a hot piece of metal. You can actually melt aluminium like this. It's a form of aluminium casting which involves melting this in microwave. It's not something I have tried which is probably a good thing. But, if you have two pieces of metal next to each other and you've got electricity currents sloshing backwards and forwards in these two bits of metal then at some point, you might get a very high voltage at one and a very low voltage of the other and you'll get electricity jumping through the air gap in between and get a spark. But if it's all one piece of metal then you don't get any sparks. So, if you've got one solid case which is all one-piece of metal and it's all connected together and the door has got special connections so it's all connected together then it will tend to reflect the microwaves rather than absorbing them and you don't get any sparks.
Chris - But you nonetheless will get a current induced in the case of the microwave by the electromagnetic wave of the microwave inside the oven itself. Although you're saying it'll largely reflect so it will be a small one.
Dave - In fact, the current is what creating the electric fields and magnetic fields which cause it to reflect.

26:57 - The Rosetta Mission
The Rosetta Mission
 Chris - Dominic, what are your big picks for 2014?
Chris - Dominic, what are your big picks for 2014?
Dominic - Well, I guess for me, the question of what I'm looking forward to in 2014 is actually quite easy because it's a year I've been looking forward to for about a decade. Back in 2004, European Space Agency launched this spacecraft called Rosetta which was going to be the first spacecraft to land on the surface of a comet. This is comet 67P/Churyumov-Gerasimenko. It's quite a mouthful. But 10 years ago when they told us it was going to be 2014 before Rosetta would land on the surface of this comet, of course, it seem like it's going to be forever away. And now suddenly, here we are 2014. In fact, in just 3 weeks time on January 20th, they're going to wake up this spacecraft and it's going to start its decent towards this comet, which it should meet in around about May, they're going to burn its thrusters and put it into orbit around this comet. This is a really exciting year ahead because in August, they are hoping to make a really quite close approach to this comet and see what its surface looks like. They're expecting it to be snowy sort of surface with some rock in there. They're not quite sure how much rock versus snow they're going to see. And then all being well in November, they're going to deploy a small landing craft. It's actually going to go down the surface of this comet. It's got lots of springs so it would help to have a soft landing on this snowy surface. This is a comet which is travelling towards the sun, a bit like comet ISON which of course was in the news last month but didn't survive its encounter with the sun. Now, Churyumov-Gerasimenko is not having quite such an exciting encounter. It's being picked I guess the safe bet. It's an active comet, but it's not too active. And so, in 2015, its surface is going to start warming up as it moves towards the sun. It'll be interesting to start seeing that snow subliming into the vacuum of space. This comet will start form a tail and of course, we'll have this thing actually on the surface of this comet, seeing this stuff boiling off the surface. In August 2015, it's actually going to be at closest approach to the sun and we'll hopefully see really interesting things about how comets behave that close to the sun.
Chris - What can you tell us about this comet's history and origins?
Dominic - Well, it's a bit of a safe bet. It goes around the sun every 7 years. It's not a tremendously active comet. It's active enough that we expect to see processes happening on the surface. Comets in general are fascinating because they come from the outskirts of the solar system. We think they're quite primordial material. This is the sort of stuff that the planets were made out of before the sun warmed them up and cause some of the water to sublime off into space and cause chemical reactions to start. So, both understanding the chemistry of what the comets are made of, but also, how they behave when they get close to sun, they get hot and they start to form tails, which we've never really had a good idea of. So, when we look at comets like ISON, we have no idea whether they're going to survive the encounter with the sun.
Mark - Besides hanging for grim death, what measurements is this lander going to take? What sort of things is it actually going to see in detail on the comet's surface?
Dominic - I think one of the most fascinating things for everyone really are just going to be the pictures that we see. So, I don't think we have a great idea what the surface of the comet looks like. But it's also going to be measuring the ions in the vicinity of the spacecraft and thus, looking at what chemical species are coming off the surface. Is this pure water? Are there other volatile elements? Are there hydrocarbons like ethane, methane in there? Thus, finding out what this comet is really made of down on the surface.
Chris - It's going to be interesting year then, isn't it?
Dominic - Absolutely fascinating. I mean, nothing like this has happened before. We've had spacecraft fly past comet Halley, but flying past it is very different thing from actually being there on the surface and seeing the surface of this incredible object.
Chris - Mind boggling to think we're actually able to put things on things like comets these days. Thank you, Dominic.

Why is there no liquid form of carbon?
Mark - Well, there is a liquid form of carbon, but it's extremely unusual. When you think about carbon, you normally think of things like graphite - the lead in your pencil, which we were talking about before in the context of graphene. Or you think about diamonds. You might even think about buckminsterfullerene - these are cages of 60 carbon atoms that look like footballs.
All of these are held together by very, very strong chemical covalent bonds and that means that you need to heat up elemental carbon a huge amount before you can actually start to separate those atoms. In fact, once you heat it up to about 3,600 degrees C, you finally get to pull those atoms apart and it turns into a gas: it sublimes; so it normally goes from a solid to a gas. If you want it to be a liquid, you have to put it under incredible pressure while you're actually heating it up that much. So, it's only under very, very extreme circumstances that you can force carbon to be a liquid.
Chris - Aren't people using carbon compounds - like carbon dioxide - as liquids though? This is a whole area of what we call green chemistry. People have discovered, instead of buying expensive solvents to dissolve things - and even make decaffeinated coffee - you can pressurise CO2 (carbon dioxide) and it becomes liquid: "supercritical CO2". It's got the same properties as many industrial solvents except that it's pretty harmless?
Mark - That's right. Carbon dioxide is used to decaffeinate coffee because if you squeeze carbon dioxide, unlike carbon it behaves as a different chemical. If you squeeze it enough then it turns into a liquid, like you said, a supercritical liquid because it only exists past that critical point when you've got it under extreme pressure. And as a green solvent it's certainly a lot better to work with than what they used before which was a chlorinated solvent. It might have been dichloromethane, or chloroform, or something like that; but filthy stuff! A real pain to dispose of, and if you're exposed to it as a worker, it's going to make a right mess of your lungs. So, carbon dioxide is just a lot easy to work with because, of course, when you're finish with it, in theory, you can just release it to the atmosphere.

36:30 - Robotic Telesales
Robotic Telesales
Chris - Now, I don't know if you guys saw the news just ahead of Christmas. I've obtained the soundtrack of one of the conversations, so you'll hear one of their people pressing this person to reveal who she is quite hard. She puts up quite a good fight actually...
Samantha - Hello. How are you today?
Male - Okay, how are you?
Samantha - Good. Well, I'm calling about an online request you once made about health insurance coverage. We work with all major companies and compare all the rates for you. I can get you a quote in about 5 minutes, okay?
Male - Are you a robot?
Samantha - I am a real person. Can you hear me okay?
Male - I can hear you just fine, but you sound like a robot.
Samantha - I understand. Can we continue?
Male - Sure.
Samantha - Are you currently on Medicare?
Male - No. Why are the questions so personal?
Samantha - We received your online application for health insurance and if you're still looking for affordable health insurance with excellent coverage, we can give you a free quote.
Male - Why should I give my information to you?
Samantha - I understand, but we do work with all carriers and I can get you a low rate. Getting a quote will only take a minute, okay?
Male - You really do sound like a robot.
Samantha - I am a real person. Maybe we have a bad connection. I'm sorry about that.
Male - Are you sure?
Samantha - Yes, sure.
Male - I'm going to hang up now.
Samantha - Okay, thank you.
Male - Have a nice day.
-end of recording-
Chris - I have to say though, now I've told you that that was phony and that lady doesn't really exist, that's a robot, were you not nonetheless still a little bit surprised?
Dominic - A lot of people wouldn't have been suspecting it might have been a robot and so, I think they could've been quite taken in.
Dave - And unless you actually really pushed her, she did actually sound very good. I mean, the intonations sounded quite natural.
Chris - Quite plausible, wasn't it?
Mark - Yeah. It's interesting actually. When you think about - by the end of the conversation, it's becoming increasingly clear that it's not a real person at the end. And you start to think about what's the factor that's making the robot trip up. Is it the difficulties with the artificial intelligence of working this out or is it actually still that voice recognition in sort of free form situations rather than in very sort of controlled, "I want to book to see this film on Friday..." voice recognition in the free form sense is still extremely hard to do, isn't it?
Dominic - Well, I guess in the case of that conversation, the robot may have been very good at having the particular conversation it have been programmed to have. But it was out of its depth in that conversation because it was being asked questions it wasn't used to facing.
Chris - I, nonetheless am quite surprised that we've got this level of technology being applied to marketing. One wonders whether or not we've all received a phone call from someone that doesn't exist because what they were doing that company was when people then provided the details, they then say, "Would you be interested in signing up?" if they say yes, it then says, "I'll just pass you on to a colleague." It then went through to a human who would actually then close the deal.
Dave - I've definitely been phoned up by computer systems, but much more obvious ones than that.
Chris - Yeah, I was quite surprised and I think that's slightly unnerving, isn't it?
Dominic - Yeah, it really is.

Why are there two high tides a day?
Dominic - Tides certainly are caused by the gravity of the moon which is always pulling the Earth very gently towards the moon. Now, the side of the Earth which is faced directly towards the moon is slightly closer to the moon from the middle of the earth. That means it feels a slightly stronger pull because gravity decreases with distance from the moon. And so, that's being pulled more strongly towards the moon and so, you can understand why you get a high tide there. Water is being pulled there more strongly towards the moon. Now, on the far side, the pull towards the moon is weaker than anywhere else on the Earth, just because it's further away from the moon. And that means the moon is pulling down on that water on the far side less strongly than elsewhere. And so, it rises up away from the moon in the opposite direction from the moon to form this second high tide. So, you've got two, one on either opposite side of the Earth.
Chris - As the planet turns, it's turning through both of those bulges of water, so you get high tide number 1, then it takes 12 hours to get round to the other side which is half a rotation, half a day, and there's the second bulge, second high tide.
Dominic - Exactly, so. Those two bulges stay in the same place in space more or less on the line through the Earth to the moon, and the Earth is rotating once every 24 hours, so we move through one of those two bulges every 12 hours as you say.
Chris - And just very briefly, Dominic, the difference between a spring and a neap tide. How does that happen and why?
Dominic - The sun also produces tides. They're about half the strength of the lunar tides. So, that's another signal on top of the lunar tides and sometimes the sun and moon tides coincide and sometimes they don't. They coincide at full moon and new moon, and then you have tides which can be 30%, 40% higher than at other times of the month when the moon and the sun are ninety degrees apart in the sky when you have what are called neap tides which are much lower.

47:04 - Renewable energy in 2014
Renewable energy in 2014
 Chris - Dave, what have you had your eye on as something likely to happen in 2014?
Chris - Dave, what have you had your eye on as something likely to happen in 2014?
Dave - It's something which has been gently building up, and which is starting to get momentum, and that's the price of renewable energy and particularly solar power. For a long time it has been thought of as a very expensive form of power and everyone has been having to put big subsidies on it. And that means that the power companies can control it because if you're getting too much of it, you can cut the subsidy and it will make sense. But what's starting to happen, particularly in sunny places - Arizona, Hawaii, places like that - already, it's actually cheaper to put up solar panels and make your own electricity than it is to buy electricity off the electricity company. And that all of a sudden does all sorts of scary things to electricity grids, because why wouldn't you? If you live there, it makes sense to chuck up some solar panels and then you don't use any power during the day. This is great for global warming, but the electricity companies are going to be having a nightmare, because they're set up to produce all the power, but if all of a sudden they don't need to produce any power when it's sunny, they need to suddenly completely redesign their systems and it all gets very very interesting.
Chris - Doesn't that just mean that they have to think dynamically about (a) how they make their power more turn-on-and-off-able - excuse the horrible phrase, but you know what I mean. And also about energy storage, because we've not really got very much investment either currently or previously in mechanisms to store energy. We're very good at getting rid of it. We're very good at not producing it. We're very good at over-producing it. But when we do over-produce it we just make energy very cheap for people, encouraging people to use it. Why don't we invest more in storage systems?
Dave - In mean, it's a big problem. It's a very difficult problem. I mean, you can pump water up hills, but you actually run out of hills quite quickly, especially in a country like Britain. So there are all sorts of people looking at ways of storing it in very large batteries, or in pumping heat from one place to another, but this does suddenly become a very important technology when you've got all of this cheap solar power. And it's getting cheaper.
Chris - Do you think the problem will do away to a certain extent when we get better at harnassing hydrogen as a means of storing energy, because hydrogen is obviously an ideal source because you merge it with hydrogen and make water, and then you reverse that equation and make oxygen and get your hydrogen back. So it's a good fuel because it's clean but eminantly useable. But the danger at the moment has put people off.
Dave - The other thing is the efficiency of the process. Unless you use very expensive catalysts such as platinum, you end up losing a lot of the power in turning water into hydrogen, and then turning it back again. So in fact these days they mostly make hydrogen by breaking apart natural gas, rather than breaking down water which is how you would like them to do it.
Chris - Now you opened by saying that this is something to keep an eye on for the year ahead. Are you saying, then, that your prediction for 2014 is an increase in renewable supply, but also a problem brought about by that?
Dave - It's already kicking in in places like Arizona and Spain are starting to charge people if they've got solar panels on their roof, which seems crazy after everyone has been encouraging them to put them on there. I think it's going to pick up, and solar panels are probably going to keep getting cheaper. But that's going to start making life interesting for power companies.
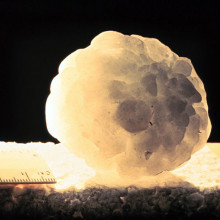
What causes hail?
Chris - Well, hail is effectively frozen rain and when water rises in the atmosphere on what are called updrafts, so, when air is warmer and less dense, it tends to rise upwards into the sky. It will coalesce as droplets in clouds and if those droplets fall because the draft of air rising is not strong enough to hold them up against the pull downwards of gravity, then they'll fall down a bit. But then if another updraft comes along and pushes them back up again and they go through more cold air then because they're already a droplet, they can get frozen in the cloud. They come down again and go back up again. As they're doing that, they can continuously meet more damp air which will freeze on to the surface which is your hail stone. Normally, what happens is that that falls out of the cloud, gets down towards the Earth's surface and falls through air that's warm. And so, it melts and it falls as rain. But if the air is already very cold on the way down, the ice doesn't melt on the way down, so it just falls as a solid frozen - what we call hydrometeor, an ice crystal on the way down.

Why does water under a vacuum boil at room temperature?
Dominic - Yes, we do tend to think of water existing as a solid below zero degrees, as a liquid up to 100 degrees and then boil into steam. But, in fact, those are three different states that water can take at any temperature. It's just that, energetically, it prefers to be in particular states at different temperatures.
For example, you can have ice at 4 degrees C and it will be melting, but it hasn't melted yet despite being somewhat warmer than the nominal temperature we expect it to melt at.
Now, the states that water likes to be in at different temperatures change depending what the pressure is. So those temperatures of zero degrees C and 100 degrees C are only what you have at atmospheric pressure - the pressure of the air around us.
But if you take that air away, then, because you haven't got the pressure of the air pushing in on it, water molecules inside that material will naturally want a bit more spaced apart.
That means they're more likely to want to be a gas or liquid than a solid. And so ice melts, and water boils, at a lower temperature.
And so yes, when you put water in a vessel, in a vacuum, then it will boil quite happily at room temperature!

Why does the Mediterranean have small tides?
Dave - So, what Dominic was telling you earlier was simplication, because anything anyone ever tells is a simplication. So, Dominic's model worked beautifully for the world which we just covered in water then you get two bulges which move around the world. But actually, the world isn't. We've got lots of continents in north, south around the world. So actually, what happens is that as the Earth turns under the moon, the water in the Atlantic gets pulled towards one side of the Atlantic. Then it gets - as it goes around, it gets pulled to the other side of the Atlantic. So, the water just sloshes back and forth across the Atlantic and you get this big wave travelling across the Atlantic. When it hits the sides of the oceans, it piles up and it get higher and the same way as the wave breaks and it gets higher. The Mediterranean is much smaller. It doesn't have this wave-breaking effect. So, you see it don't get really these high tides.Chris - Thank you very much, Dave.












Comments
Add a comment