X-ray to MRI: Unpacking Medical Imaging
This week, we’re unpacking ultrasound, imagining MRI and pondering PET scans, as we ask - how does medical imaging actually work? And what future innovations are waiting in the wings? Plus, we hear from the group that’s tracking mutant coronaviruses...how do they find new variants? And a new battery for electric vehicles that can charge in 10 minutes!
In this episode

00:59 - Finding mutant coronaviruses
Finding mutant coronaviruses
Sharon Peacock, Cambridge University and COG-UK
Across the UK, coronavirus cases are now thankfully falling. But the surge in coronavirus cases we’ve been suffering was triggered in the first place largely by the combined effects of winter, which helps viruses to spread more efficiently, and a more transmissible "new variant" of the virus thought to have emerged first in the southeast of the country and which may, according to UK Chief Scientist Patrick Vallance speaking at a recent Downing Street Press Conference, also carry a slightly higher mortality rate than the parent virus.
Katie - Other countries have also detected mutant coronaviruses that have enhanced abilities to transmit, and possibly sidestep our vaccines. This has prompted the government to put in place measures to block travellers entering the UK without a prior negative test. Transport secretary Grant Shapps outlined these measures recently...
Grant Shapps - What we're trying to do now is stop a new variant, which isn't established here, from coming here. So we've done this today and I notice the Canadians - the prime minister Justin Trudeau there - has done exactly the same thing as us. Also introducing a 72 hour test in addition to the quarantine measures which remain in place.
Adam - But one of the reasons why we know about the new UK variant, and found out so quickly, is thanks to a consortium called COVID-19 Genomics UK, or COG-UK. It's led by Sharon Peacock from the Dept of Medicine at Cambridge University. She told Chris Smith how the initiative works, and, interestingly, speculated that one reason why covid is surging in so many countries is that variants like those we've picked up here might be a lot more common around the world than we think…
Sharon - COG-UK is a network of around 300 people who are scattered all around the country, to sequence the cause of COVID-19, the virus SARS COV-2 virus, to track important changes in the virus, as it evolves. So far in our efforts, we've sequenced around 200,000 genomes in total. Now to try and contextualize that, we have contributed around just under half of all the global genomes that are available for this virus at the moment.
Chris - And when you sequence the genome, what's actually involved in that and how do you get access to those samples in the first place?
Sharon - Well, we get samples after people have been tested and so, the diagnostic test, this is the PCR assay. We take the waste from that has been extracted from the sample for that test and we use it to prepare some further material that can be sequenced in a sequencing machine. The end result is that we have the genetic information for that virus, which is around 30,000 different points in the genome.
Chris - So 30,000 genetic letters long, it's quite big, what do you then do with that information? Because you basically have ended up with a giant document, which is 30,000 letters long. What do you do next?
Sharon - We're interested in two particularly important things. Has the virus developed a mutation - that means a mistake or a combination mistakes, actually more often a constellation of mistakes - that could change the way that the virus actually behaves and interacts with humans. The other way is that we compare the entire genetic code with that of other viruses and we can use that to look at issues such as has there been an outbreak.
Chris - So when you've read the genetic code for a sample and maybe you could tell us also how you choose where around the country you sample, or is it just random? Do you then basically line up your new sequence you've got from that sample with the sequences that you've read before and ask, is this one, any different?
Sharon - There were two parts to your question. The first is where do we get the samples from and how do we choose them? We try to cover the entire country. We aim to get samples from people who have infection and who are in the community and don't need to go into hospital. We also obtain viruses from people who have been admitted to hospital because you want the entire spectrum. And then when we have a full genome of a virus, we will compare it to other viruses, bit like a family tree. We also have software tools that really help us identify particular points in the genome that are of concern to us because for example, they could be related to a greater transmissibility spread between people or possibly the way they interact with the immune system.
Chris - And when the new variant that a lot was made of this around Christmas time but in fact, it began to emerge much earlier than that didn't it? How did you first notice that and how did you then track it? How did you realise it was significant?
Sharon - It was only looking backwards that we realised the very first sequence was sequenced on the 20th of September last year. And it's actually looking for new variants is like looking for a needle in a haystack because there are thousands and thousands of new mutations in the virus. And so looking for one that's important can be very challenging. It was only really in mid to late November when it really became clear that there seemed to be a spread of cases in the South of England. Whereas there wasn't spread in other parts of the country that were under similar lockdown rules. There are a range of explanations for that, but one of them is the virus could be somewhat different in its behaviour. So at that point, people that have information on populations and clusters of cases realised that the two could be related. It seemed to be an increasingly transmissible virus linked to a very specific genome.
Chris - And do you think this is going on in terms of making these sorts of very transmissible viruses all over the world? And it's only because we have you that we know about it, or are we a special case?
Sharon - I don't think we're a special case at all. The force of nature is being seen across the world, but you're only going to see what you seek. And so we, because we are sequencing around half of the world's genomes, are likely to detect those mutations. But the similar mutations that are going to be important in terms of transmissibility and vaccination are very likely to emerge from around the world. And in fact, we're seeing that sort of pattern happening now.

07:12 - The strategy to ease lockdown
The strategy to ease lockdown
Gabriel Scally, Independent SAGE
Here in the UK, we remain in a lockdown with many people unable to work, we're banned by law from socialising, festivals like Glastonbury and many sporting events are on hold. In England, the legislation underpinning these rules is in force until March the 31st, but many are asking what is the roadmap for easing this third and - hopefully final - lockdown, and what provisions must we have in place by the time it's over? Public Health physician and independent SAGE member Gabriel Scally told Chris Smith how he's reading the situation…
Gabriel - I think our position is really very serious. Certainly the virus infection is still at an extremely high level. It is coming down thankfully and so it should do because of the level of restriction, but it's got a long way to go and we really want to see it come down just as fast as it went up, because every day it lingers at a high level, it causes more cases. So that's good it's coming down, but needs to come down faster.
Chris - What do you think that the risk is of stepping back too quickly? Because Chris Whitty chief medical officer for England made this point several times in last week's Downing Street press briefing, where he said, we need a managed retreat back from where we are. So we don't release things too quickly. Obviously the question he was being asked was about easing lockdown restrictions. So what do you think is the correct way from where we are now to get out of this?
Gabriel - Well we do absolutely need to get the virus under control, keep it under control and preferably keep new variants out. I think if we did those three things, getting it down through the restrictive measures is absolutely important. Keeping it down really depends on a raft of other measures being done properly, things that haven't been done properly, such as putting in place a really good find, test, trace isolate and support system. And we haven't really explored what we can do to improve ventilation because that is a key preventive key public health measure - ventilation in our schools before they go back, ventilation in our workplaces, in our retail places, all of the places behind a door where that door would be much better open and the windows open as well.
Chris - What do you think about the current vaccine situation? Because obviously when 2021 was born and we launched in with not one, but now two vaccines - so we're fighting the virus on two fronts - and I mean, you have to acknowledge, they've got to more than 4 million people very, very quickly with these vaccines, but is this the right approach and, and what should be our expectations from this vaccine campaign?
Gabriel - I think getting the most vulnerable people, those really elderly people and also those with serious other conditions troubling their health, getting them vaccinated as quickly as possible is extraordinarily important. But it's also really important to make sure that we get a really high uptake of the vaccine. I know from my own personal experience of running vaccination programmes that getting the first 50, 60, 70% even is relatively simple. Getting it up 80 to 90% and over 90% in an adult population is extraordinarily difficult, but that's what we have to really concentrate on doing. So I'm heartened by the progress so far, but it's still got a long way to go and one of the things we must not do is take our foot off the pedal here. I'm very clear that people must understand just because you've had one dose of a vaccine, doesn't mean that you are immune from the virus. It will, over a period of weeks, give you a certain amount of immunity, but everyone just still has to retain the same social distancing, the same safety measures essentially to keep themselves safe, particularly if you're a vulnerable person.
Chris - Is your expectation then that this virus is not going to go away. It's not something we can eliminate. Like we have eliminated polio from many places, measles from many places, sadly, not our own country anymore. And that basically we need to make plans for expecting this to be with us for the foreseeable future and therefore we need some way of suppressing it and suppressing its effects, but accepting it's always going to be there.
Gabriel - Well, both of those things, they're not incompatible. Many countries in the world have achieved elimination. Elimination doesn't mean that there are no cases at all. It means that there's no local transmission, that the infection doesn't roll on in a community. Particularly Australia, New Zealand and plenty of Asian countries who have got to zero COVID and kept to zero COVID, they're still picking up cases, but they're picking them up in the managed isolation facilitates that I think we should have. So it is possible to have the virus eliminated in the sense that there's no longer any local spread, but lots and lots of countries will not have the ability to do that and therefore, I think it's very likely that the virus is going to be with us for some time. And the whole way of thinking about this may change, of course, at any moment, if the virus starts to produce variants that somehow circumvent the effect of the immunisation, then we're in difficulty. And it may not be as easy as it is with the influenza virus to find those variants, produce a vaccine for them, and then vaccinate the whole population. We know now, what a difficult task that is. So I think we're going to have to continue for some time to have a mixture of measures. If I had my choice, I'd rather live in one of those countries that had zero COVID and was operating normally, rather than a country that was trying somehow to live with the virus.

13:45 - 10 min charging time for new e-car battery
10 min charging time for new e-car battery
Chao-Yang Wang, Pennsylvania State University
Governments in many countries are on a drive to encourage the use of electric vehicles to cut pollution. In the UK we have the ambitious target of ceasing the sales of new petrol and diesel cars before 2030. The problem is that the present generation of electric vehicles are very expensive, and most of the cost is the battery. There’s also the disincentive of the slow recharge times, that can be hours in some cases. So we welcome news from engineers at Pennsylvania State University who have developed a new battery technology for electric vehicles that - they say - is cheap, gives you decent range, and can recharge in under ten minutes! They’re using a technology called lithium iron phosphate that the industry had largely moved away from, because at regular temperatures it stores less energy than other types of lithium batteries. But researcher Chao-Yang Wang has come up with a couple of clever tricks to make it work - as he told Phil Sansom...
Chao-Yang - We created a low cost battery that can be rapidly charged, and also significantly reduce the battery cost so that, you know, average people can afford electric vehicles.
Phil - It's a pretty impressive claim. How have you done all of this?
Chao-Yang - Well, we have been working on batteries for 25 year,s and in the last five years or so, we made a major breakthrough in terms of adding this self-heating function into the battery.
Phil - Self-heating. Does that make it charge quicker because the chemistry becomes more efficient or what?
Chao-Yang - Self-heating is basically accelerating the chemical kinetics, the movement of lithium ions between the two electrodes, anode and cathode. We typically would raise the cell temperature to about 60 degrees Celsius.
Phil - How does this heating process work? Because this must take a bit of energy itself, right?
Chao-Yang - Sure. So what we did is basically, we invented a very elegant self-heating structure, which consists of a nickel foil, very thin, 10 micron thickness, nickel foil.
Phil - Nickel metal.
Chao-Yang - Yes. And then insert that into the battery, because the battery has the energy. So you really don't need any external heat source, this well cause internal heating, extremely efficient and fast.
Phil - Isn't the problem with these car batteries that previously, when they've heated up, you get this sort of catastrophic chain reaction? And that's why you hear stories of car batteries, catching fire, little explosions, that kind of thing. How have you avoided this enormous problem?
Chao-Yang - Yeah. You have an excellent point, and typically battery materials are afraid of the high temperature. So the trick we play here really, is to maintain the elevated temperature only for about 10 minutes per charge. So think about it, after your charge, you have about 200 to 300 miles. So for every 200 to 300 miles, you only have 10 minutes exposure to this elevated temperature. So you have limited material degradation, and that is how we balance the ability to fast charge due to high temperature, and suppressed material degradation at the high temperature.
Phil - What about this other claim that you've told me about that it's, you know, pretty low cost, where's that coming from?
Chao-Yang - So once you have the fast charge ability, you will be able to downsize the battery on your vehicle, and smarter batteries, lower cost. We also use low cost materials, and we use a lithium iron phosphate as a cathode, which is thermally, extremely stable and safe. So with these two aspects, smaller size, and the use of cheap materials, we will be able to drive the cost down to about $3,500 per vehicle, on par with gasoline engines.
Phil - So all good news. Have you put this in a car yet?
Chao-Yang - No, not yet. I think the next challenge is working with automakers and battery manufacturers.
Phil - Okay. Do you know it will actually work in a car?
Chao - Yang - Yeah, I think so, because this low cost thermally modulated battery is fundamentally based on the self-heating structure, which has been used to produce an earlier product, called the All-Climate Battery. And that earlier product, the All-Climate Battery, has been used in cars.
Phil - Okay. How long until I can buy this?
Chao-Yang - My batteries, I think will be in the marketplace, in around three to five years.
It’s unclear whether the high temperature charging will degrade the battery over the lifetime of a car, so we’ll keep an eye on that.
18:48 - Context-dependent smell in butterflies
Context-dependent smell in butterflies
Kelsey Byers, John Innes Centre
Now imagine strolling with your significant other through a park, and stopping a moment to gaze upon a beautiful butterfly…. Well I’m afraid that’s where the romance stops. Because news is out this week that male butterflies from a species called Heliconius melpomene, have evolved to produce a strongly scented chemical in their genitals, which they leave behind after sex with females, to deter other males from pursuing their mates. Lovely! And strangely, this same chemical is identical to one produced by plants to actually attract butterflies. So it’s either gross or great, depending on context. Katie Haylor spoke to Kelsey Byers from the John Innes Centre in Norwich, one of the scientists behind the study...
Kelsey - This is a chemical called beta-ocimene, and it smells kind of green, a bit medicinal, a little bit flowery. It's a little hard to describe.
Katie - You've been studying the genetics of this. So what experiments did you do?
Kelsey - We did a couple of different experiments. The first was, we understand a little bit about how this chemical is produced in plants, and also in some types of insects, or how similar chemicals are produced. And so we looked at the biosynthetic pathway to try to identify genes that might be important for the production of this chemical. We also took advantage of the fact that a related species, Heliconius cydno, doesn't produce the chemical, and used a genetic mapping technique to try to identify the genetic origin. So we found some candidate genes, very exciting. And then what we did is we looked at their expression. We're looking for genes that are expressed, as in many copies of the genes are transcribed, at high levels in the male abdomen, which is where the chemical is made. And we found a couple of candidates. When we put those candidates into bacteria, we found that one of those candidates in particular produce the chemical that we're looking for. And so that's how we identified the gene.
Katie - So what does it mean then?
Kelsey - The butterflies produced this chemical, and it's beneficial for male butterflies because it means that the females don't remate, and therefore all of the offspring of that female, probably for most of the rest of her life, will be from that male. It also benefits the females a little bit because it means they stop being harassed by other males, because the other males can smell and go, Oh, she's already mated. She's not going to be interested, but it also might cost the female if she's mated with a less good male. And now she would like to mate with a better male, but that better male is scared off by this chemical compound.
Katie - So is it the case that this same chemical is being made by these insects, but also by plants?
Kelsey - Absolutely. It's actually one of the most common volatiles that's produced by plants. And if you were to analyse the smell of different plant species, you would find this very frequently. So it's a little bit strange. We were curious, if it was produced by the same mechanism in both plants and animals. And it looks like it's actually produced by very different mechanisms between plants and animals. So between the flowering plants and the butterflies.
Katie - So hang on, if this chemical is attractive or repulsive, depending on context, you know, mated with a female or looking for a plant, how do the other males know whether to be grossed out, or think it's great?
Kelsey - The word you said, context, is absolutely the answer. So it depends on the context. If they smelled this chemical in combination with a female, they go: "Oh, the female has been mated." It's also at much higher doses in the butterflies than it is in the flower. So I can smell it a little bit in a flower. But if you have a female butterfly and a net, wow, you can really smell the amount of chemicals. But a lower dose of the chemical in combination with signals that look like a flower, would make the butterfly go: "Oh, this is food. This is great." So it's all about that context.
Katie - And so you're talking about butterflies in the net. Has someone done the behavioural experiments to back this up?
Kelsey - Absolutely. So it turns out that if you paint this compound onto the abdomens of female butterflies, the males will actually avoid them. So we, in this study, we looked at the genetics, but the behavioural effect of this chemical was actually already known.
23:23 - Discovering X-rays
Discovering X-rays
Katie Dabin, Science Museum
Anatomists and doctors have been looking at and illustrating the body since ancient times. But having the tech to image the inside of the body while a person is still alive arguably comes about with the discovery of X-rays, which happened in the late 1890s. Katie Dabin, is curator of medicine at the Science Museum in London. She took Katie Haylor on a virtual tour of some of the museum’s relevant relics...
Katie D - When we think of x-rays, maybe when we've broken a bone, we go to hospital, it's all very high-tech medical equipment. But what you're looking at here is essentially a mahogany wooden table with a very strange looking coil on top, and then a wooden tripod with an oversized looking light bulb attached to it, and at the back the equivalent of a car battery. And this was the kind of equipment you could quite easily get hold of in the 1890s and produce x-rays with. And this was built just a few months after Wilhelm Röntgen, the German physicist who discovered x-rays in 1895, surprised the whole world with this amazing discovery. People began to experiment and build their own kit. You can imagine the scary levels of radiation people were sort of getting exposed to at that point.
Katie - So what's going on with this kit? How do you make an x-ray image?
Katie D - All the other kit essentially starts creating the right kind of current and voltage that you need to produce that electrical charge. And then when you come to the oversized light bulb, this glass vacuum tube, electrons get emitted from one end, the cathode, and they go across the vacuum and hit the positive end, the anode. And when those electrons start hitting that anode that's when they start emitting these higher energy radiation x-rays. Röntgen the German physicist didn't know what these were when he first started observing them. So that's why he termed them X for unknown.
Katie - So how do you get an image of something? Say a part of the body?
Katie D - The high energy x-rays, they act like lights. Sometimes light gets reflected, or sometimes it gets absorbed by different materials. X-rays are able to pass through a lot of our soft body tissues, and that's when we see the sort of shadowy dark areas on a body x-ray, but they get absorbed by our bones. So that's when the bones show up as the kind of lighter areas on an x-ray.
Katie - And so what was the German physicist that you just mentioned, Röntgen, what was he up to then?
Katie D - Like a lot of other physicists at the time, Wilhelm Röntgen was really interested in exploring electrical properties of these vacuum tubes, these oversized light bulbs. And he was doing all sorts of experiments, especially these ones called Crookes tubes. He started observing, even though he'd covered up all the light-emitting properties of the tube, he had some sort of photochemical materials on cardboard nearby, and then he started noticing this weird fluorescing glow in his lab. He started observing and then began to carry out experiments to understand what was causing this weird fluorescence. And then he realized that the tubes themselves, even though he was covering them up so they couldn't emit any light that we would see, that there must be some rays coming from these tubes that was causing that material to fluoresce, and gradually started experimenting. And then, by accident I think, put his hands in front of the rays and ended up capturing an image. And when he first showed an image of his wife's hands, I mean, she saw the sort of skeletal image and looked aghast and was like "Oh, I've seen my death!"
Katie - I can imagine if the only skeletal images you've seen are of dead people, that sounds like it could be quite disconcerting!
Katie D - Absolutely. I mean x-rays are transformative in the ways people were seeing things at the time. This is the Victorian period, generally they were seen more as a new form of entertainment. It became very fashionable to get your own sort of x-ray image of your own hand or foot. But increasingly and gradually, we became aware of the real harmful health consequences of the radiation that x-rays emitted. It became much more medicalised and industrialised and very much out of the hands of your regular everyday person. And pretty quickly within the Boer war, which was around about 1896 as well, and then into the first world war in particular where the technology had improved so bit more reliable, x-rays were carried onto the frontline and they really proved themselves to be useful in identifying bullet wounds or different fractures or wounds. And gradually it became such an important part of medical practice from there on in.
Katie - So how do you get from x-rays to something a bit more sophisticated, like a CT scan?
Katie D - That's a technology that still makes use of x-rays. So rather than more like a photographic image, what's really clever about scanning technologies like CT is that you can make these 3D slices all the way through the body in all sorts of different ways - up, down, through sideways, horizontal, and reconstruct a whole 3D image through the use of computers.
Katie - Science has come an enormously long way from the late 1800s to now in 2021 with the medical imaging that is possible. Can you summarise the sort of public perception journey through that time?
Katie D - From its birth with x-rays - technology that could be used for entertainment - it wasn't conceived as just a medical or an industrial tool. You would even find x-ray machines within shoe shops and people could go in and try shoes on under these fluoroscopes called Pedoscopes and see their toes wriggling around in real time. Equally, along with that sort of shifting and increasingly medicalised sense of medical imaging, what really partly drove that was the risk attached with the radiation of x-rays. So increasingly we became really aware of the health risks. And so increasingly as the regulations got tightened and also the kind of practical applications really became much more seen within either medicine or industry in different ways, we've come to understand these technologies predominantly within a hospital setting and definitely not one for the home.
How ultrasound scans work
Phil Sansom explains how ultrasound scanning works...
An ultrasound is a type of scan that you probably know as being useful for checking on unborn babies. It’s called ‘ultra’ because it uses sound waves at ultra high frequency - way higher than a human can hear - to get an image of a specific area of your body. The sound waves echo off the boundaries between different tissues, and a probe picks up the echoes and turns them into an image in close to real time. A bit like a bat using echolocation to observe its surroundings!
Ultrasound is not just for pregnancies. It’s great at monitoring most organs through your skin. And on top of these “external” ultrasounds, there are also internal ones, such as transvaginal scans that check on your ovaries or womb; or you could have what’s called an endoscopic ultrasound, where the probe is attached to a long tube and goes down your gullet, to spy on your digestive system.
Ultrasound scans work very well if the thing you’re looking at is close to the probe, like an organ that’s directly beneath the skin. They’re not very good at bones, joints, and things that contain air, like lungs.
The good news is that there are no known risks from the ultrasound sound waves. You don’t get exposed to radiation, and you don’t need a scanner the size of a room! But considerations come if you get an endoscopic ultrasound, which can be a bit more uncomfortable and cause temporary side effects like a sore throat. Luckily for these ones you’ll usually be sedated and have your throat numbed by anaesthetic.
31:16 - Combining scans to refine biopsies
Combining scans to refine biopsies
Mireia Crispin Ortuzar, Cancer Research UK Cambridge Institute
One area medical imaging is routinely used in is cancer diagnosis. And while scans like x-ray, ct and ultrasound are relatively non invasive, sometimes it is necessary to do an invasive procedure to better understand what’s going on, for instance a biopsy. In a proof of concept paper published recently looking at ovarian cancer, Cambridge University scientists say that by combining ultrasound and CT scanning in real time, they may be able to reduce, refine and maybe even one day replace the need for doing physical biopsies at all... Adam Murphy spoke with Mireia Crispin Ortuzar, a Research Fellow at the Cancer Research UK Cambridge Institute...
Mireia - Well, the first thing to keep in mind is that cancer is a very broad term. We know over 200 different types of cancer. So figuring out the type of cancer and how advanced it is is one of the very first things that an oncologist does to decide how to treat a patient. Now, imaging is used as part of that process. It's very important. It helps us see if there is cancer, how big it is and whether it has spread to other parts of the body. But, the potential of imaging to tell us the exact type of cancer that we're dealing with is limited. There has been some progress recently using computational analysis techniques, but in general, we still need to gather additional information for a complete diagnosis. So the reason biopsies are so important is that they help doctors diagnose the exact type of cancer. They're essentially very small samples of tissue that are extracted directly from the tumour with a fine needle. And then the samples are examined in a microscope and doctors can literally see the tumour cells directly, if there are any, and produce an accurate diagnosis. And having an actual tissue sample also allows you to, for example, look at the genome of the tumour cells and potentially identify important mutations for which special treatments are available. So they play a really crucial role in the cancer journey.
Adam - So then what is it that this paper has done that might ease that burden on biopsies?
Mireia - The challenge with some types of cancer, for example, ovarian cancer, is that they are extremely diverse. We know that even within a single tumour, there may be regions with different underlying biology and therefore potentially different response to treatment. And the problem is that right now that diversity is not taken into account when you take your biopsies, simply because there are no methods to do it. So the main aim when you take a biopsy is simply to extract some tumour cells - independently of where exactly they came from from inside the tumour. So what our team has done in the past is that we found that applying computational pattern recognition techniques on CT scans can help you to identify some of these distinct regions inside the tumour, which we call habitats because they have this kind of spatial distribution. And this is really exciting because CT scans are part of the standard of care for cancer patients. The great majority of patients will get them. So it's data that is very easy to get.
So our hypothesis was if we could show those habitats in real time to the radiologist who is taking the biopsy, they may be able to take those biopsies strategically, targeting specific habitats to obtain a better understanding of these really complex tumours. Now, the main challenge is that biopsies are usually taken using ultrasound imaging, which helps guide the needle. So what we have done is to design a technique that allows us to overlay a map of these habitats in real time on the ultrasound that is being used to take the biopsy, so that we can make sure that that biopsy is being taken from exactly those regions of the tumour that we are interested in.
So in this specific paper, we focused on high grade serous ovarian cancer, which is the most common type of ovarian cancer. It usually presents itself when it's already metastatic. And so we were able to look at six patients and we looked at two different sites of disease, the pelvis and the omentum. So for two patients, we looked at the pelvis, for four patients we looked at the omentum. And we were able to understand the heterogeneity of the disease in both cases. And the reason it's so important to have techniques that allow us to capture that variation is that the better we are at understanding the underlying heterogeneous biology, the better we will be at choosing the right treatment for individual patients.
Adam - We mentioned this was a proof of concept paper. So, what does this mean for actually being used on patients?
Mireia - A short scale aim is to be more strategic about the biopsies that we do take. In other words, you know, if we can only take one biopsy, can we make sure that it will be the most informative one? So for patients, the experience would be pretty much identical to now. They would still come in and have a biopsy. But the important difference is that we would be getting a lot more useful information from it. And this could be really key for cancers that exhibit this type of heterogeneity of spatial diversity that makes them so hard to treat.
If we can characterise habitats using imaging data well enough, it might be possible to eliminate the need to take actual tissue samples and therefore replace biopsies. And this is a long-term vision, which will still require a lot of work, because we need to make sure that our understanding of the biology behind the tumour habitats is highly accurate. It's guiding our way, it's our long-term vision. But I think that being strategic about the biopsies that you take is the intermediate aim that we're going towards.
How MRI scans work
Adam Murphy explains the basics of an MRI scan, and then Katie Haylor experiences one...
Adam - An MRI is a magnetic resonance imager. It is in essence, a giant, really powerful, magnet. Hospitals are always really careful to not let anything magnetic into the room while it’s on, because it would get sucked in and damage their expensive machine (as well as anyone in it!)
MRIs are particularly good at imaging the squishier parts of us. Although they don’t always give the resolution that CT would. They're also quite complicated in how they work.
Your body has a huge amount of water in it. We’re 65 % water. And that water has two hydrogen atoms, and those atoms contain protons, little particles of positive charge. Those protons have a property called spin. You can think of it a little bit like how the Earth spins on its axis. If you put a person in a strong magnetic field, the protons in all the water atoms of your body start to spin either with, or against the magnetic field.
When you blast radio waves of just the right energy, which depends on that magnetic field, at these protons, they flip away from the magnetic field. When you stop, they go back to their original positions. As they do, they give out some energy. We measure that energy, and that tells us where water is in the body, and that’s how we generate the image.
By precisely tuning the strength of the magnetic field in different places, we can get information about the different amount of water all around the body, in 3-D
MRI is particularly useful, because unlike X-Ray, or CT, or even PET, there’s no ionising radiation, so it doesn’t damage you to do it. Although it can take a bit of time, and you have to sit very still in a large loud machine.
Katie - I actually got to experience an MRI scan of my brain a few years back. The resulting image has pride of place on my desk at home. And I remember taking off any metal, as Adam just mentioned, and being given some earplugs. I took my shoes off and laid down on actually quite a comfy bed, which slowly slid into a rather large donut...
Hi Katie, how are you?
Katie - I'm pretty snug to be honest.
So are you ready to start?
Katie - I am.
Okay. So I just need you to stay very still. Okay. And then you're just going to hear some sounds and then I'll speak to you after that. Okay.
[MRI sounds].
Michael - Katie Haylor, meet Katie Haylor's brain. I just want to emphasise the specialness of this, because the fraction of all humanity who has ever lived on Earth who has actually gotten a chance to see their own brain is very tiny. And you now are welcomed to that club.

40:28 - AI helping doctors read MRI scans
AI helping doctors read MRI scans
Andrea Rockall, Imperial College London
MRI can be used to image different parts of the body, and can give really detailed information about tissues. But with radiologists - doctors specialising in medical imaging - in short supply and the difficulty of interpreting some of these scans, researchers are busily working at ways to harness the potential of artificial intelligence and machine learning to translate these medical images faster and more accurately. Andrea Rockall from Imperial College is one such radiologist who spends half the week in the hospital and half the week in research - currently, training machine learning algorithms to help doctors read whole body MRI scans. Katie Haylor spoke to Andrea, asking firstly about when someone might actually undergo a whole body MRI scan...
Andrea - Mainly this would be in patients with suspected or confirmed cancer where we want to identify the initial tumor and also sites where the tumour may have spread. One of the challenges we have is that patients with cancer may have multiple different types of scans, for example a CT or a PET-CT or an ultrasound. And the concept would be that a whole body MRI may be good enough to do the full staging of the patient in one sitting.
Katie - Oh, I see. So you're not having to translate between different technologies.
Andrea - Yes, obviously each of the different technologies has pros and cons, but whole body MRI may be able to replace multiple visits to the hospital and multiple scans for each individual patient because it gives such an excellent overview.
Katie - How have you actually trained the computer to read these whole body scans?
Andrea - The way we train the computer is by teaching it essentially what an expert does. So the first step for the whole body MRI machine learning training is really to demonstrate a whole number of scans in healthy volunteers that are labeled, so different organs are labeled. So the liver and the lungs and the heart for example, are labeled by the experts. And these are fed into a computer that then learns about what the normal organs look like.
We then take a set of scans in patients with cancer and effectively the expert colours in the sites of the cancer. So labels them as sites of cancer. And then these scans are then fed into the computer algorithm in order to train the computer to recognise the appearances of the cancer on the scan.
Once we've trained the algorithm to recognise and detect these areas of abnormality, we then test the output on new scans that the computer's never seen in order to see how well the algorithm is performing.
Katie - How well is it performing? Do you have enough data at the moment to be able to say?
Andrea - With our first study, which has just been completed, we found that using the AI algorithm output with an expert reader... essentially the expert reader performed approximately the same, but perhaps was very slightly quicker at doing the read. What we did find is that there was some improvement in the detection of cancer when you asked a more junior reader to look at the scans and diagnose the disease. So it may be that this technique will help people who are training or less experienced at reading whole body MRI.
Katie - So just to be clear, what you're saying is: your initial findings suggest that the algorithm does better than slightly more junior members of staff?
Andrea - Well, don't forget these algorithms are being read in the presence of this expert, so the expert has an overlay of the machine learning output; and their performance is compared to how they perform without the machine learning output compared to with. It's not the machine working on its own; the machine does not perform well enough at the moment to do that.
Katie - Okay. So it's very much a joint venture between an expert human and hopefully an expert algorithm.
Andrea - It is exactly that. The expert or the junior reader will be provided the scans, with or without the machine learning output, to see how that affects their diagnostic performance.
Katie - What is your hope? Is it that, with the help of some sort of machine learning algorithm, doctors will be able to read these body scans faster, or more accurately, or both?
Andrea - We would hope for both! Firstly, as the scans are going through the scanner, if there's a serious abnormality that needs urgent attention, we would like to see that those scans would be triaged quickly so that they come to the top of the list for the radiologist to report. Secondly, it would be great if areas of concern could be highlighted for the radiologist in order to help them with the read. But thirdly, if we can speed up the time it takes radiologists to read, that would be wonderful as well, since we're short on radiologists in the NHS.
Katie - Are there other areas of healthcare where we're already doing stuff like this?
Andrea - Yes, whole body MRI is at the very far end of the spectrum. Where we have successful AI outputs that are available for clinical care include looking at CT scans of the brain in patients with suspected stroke; so these are available now in the NHS, and here we can speed up differentiation between somebody who's had a bleed or a blood clot, and that can speed up the triage to treatment. And for example, in Accident and Emergency, there are AI tools which are approved for detection of fractures, for example; or something called a pneumothorax in the chest; or pulmonary embolism, where you get a big blood clot in the chest. And some of these are already available in much simpler technologies than the whole body MRI.
Katie - How long do you reckon it'll take before whole body MRI scanning, as read by a computer, would actually be in hospitals?
Andrea - I hope perhaps in the next five to eight years. But it takes this long just to really, really develop the technique to a stage where it is safe for application to patient care, and where we've actually tested it, a little bit like we would run a drug study to make sure that the diagnostic tool is working effectively and safely. Doctors are quite conservative, I would say, and they need pretty solid data with evidence of safety before rolling out. Some of the things in the slightly simpler technologies, like plain films or CT scans, will be coming through already now; but also more and more tools will be coming through in the next five years or so. The whole body MRI, I think, is going to take a little bit longer.
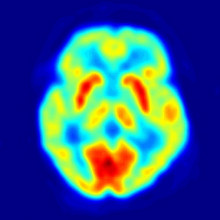
How PET scans work
Katie Haylor explains the basics of how a PET scanner works...
A PET scan, or a positron emission tomography scan (quite a mouthful) is a clever way of getting detailed 3D images of how things are working inside the body - rather than just what an organ looks like. In PET, you’re injecting a radioactive chemical that you can track - commonly a type of sugar - into someone and in detecting the radiation being emitted from the tracking chemical, you can see how well different body functions are working.
Once the chemical’s been injected, the radioactive sugar gives out a particle called a positron. When a positron hits an electron in the body, they both turn into high energy waves. The PET scanner scans the body part of interest and looks out for high energy waves. In detecting this energy (known as gamma radiation), you can build a map of what’s happening in the body.
It’s useful to look at cancers, because cancer cells are metabolically rather hungry and use sugar faster than normal cells. Taking up more of the radioactive sugar means more gamma radiation detected from that area. So on the image, the cancerous bits will appear brighter.
So if you’re injecting someone with radiation, how safe is a PET scan?
The National Health Service website explains that any exposure to radiation carries a very small risk of potential tissue damage that could lead to cancer at a later date. But the amount of radiation you're exposed to in a standard PET scan is small – about the same as the amount you get from natural sources, such as the sun, over 3 years.
48:43 - PET to image onset of Alzheimer's
PET to image onset of Alzheimer's
Justin Sanchez, Massachusetts General Hospital and Harvard Medical School
Alzheimer's Disease is thought to be caused by the abnormal build up of proteins in and around brain cells. A key priority in Alzhimers research is finding ways to get vulnerable people treated earlier, when interventions are more likely to be effective. And so understanding what happens in these protein build ups early on in the brain is a crucial question to answer. So could imaging where the protein build ups are happening early on in someone’s journey to Alzhiemers help with drug trials to treat these build ups, or potentially even diagnose people earlier? This is the hope of scientists from Harvard, including Justin Sanchez, who, having laid the groundwork with MRI has just published a paper about a new way of using PET to do exactly this. Adam Murphy spoke to Justin, asking first how the use of PET in this context compares to the use of PET to look at cancer cells...
Justin - The idea is basically the same: that you're injecting a radiotracer into the body, and then tracking it within the PET camera. But the difference here with Alzheimer's imaging is that we've designed these tracers to bind specifically to the proteins that we know build up in the brains of people with Alzheimer's, namely amyloid beta and tau. So by using these advanced PET imaging methods, we're able to now detect the early stages of Alzheimer's pathology, and also track changes over time.
Adam - Where exactly in the brain does this tend to show up? Is there anywhere in particular you're looking?
Justin - Yeah, so we know from autopsy studies of Alzheimer's disease that tau accumulation tends to follow a pretty stereotyped pattern; namely, it begins in this very specific area of the brain's medial temporal lobe that we call the transentorhinal cortex. And from there, as amyloid is also accumulating, it spreads to other regions of the brain and what we call the neocortex. And we really think it's this neocortical tau accumulation that is the bullet that really injures brains in Alzheimer's disease.
Adam - Now you looked at about 400 odd people from a huge range of ages. So what is it that you actually found out about tau?
Justin - What we applied here was a sort of automated method for identifying, in each individual person, this region of earliest vulnerability to tau accumulation. So we know to look in the transentorhinal region for the initial stages of tau, but the challenge that we face is that each person's brain anatomy is slightly different. And so what we did was basically modelled the surface anatomy of each person's brain as a kind of map; you can think of like a map of hiking trails, where you have the mountains and the valleys, and those corresponded in our data to the gyri and the sulci - the hills and valleys of the brain. And then we implemented an algorithm that would automatically identify the valley in which the transentorhinal region is found, accounting for individual differences in surface anatomy. And so we looked, as you say, across about 450 people, spanning the whole range from 20 year old healthy people to older folks with a diagnosis of dementia; and we looked along there to see at what point we observed the initial accumulation of tau. And what we found was that, even as early as 58 years old in patients without dementia or without even significant amyloid accumulation, we were already able to detect these very earliest signs of tau pathology.
Katie - Justin, what does this mean for people who go on to develop Alzheimer's? Because these buildups can occur, but it doesn't necessarily mean someone's going to get Alzheimer's.
Justin - That's exactly right, yeah. So it's still kind of an open question for us what level of tau is really dangerous. Because the autopsy data would suggest that about half of people, by the time they're 50 years old, have some degree of tau accumulating in their brain; but obviously not everyone is going to go on to develop Alzheimer's. So we wanted to know in this study - and we followed people over over two years to track the changes longitudinally - we wanted to know what basically were the best predictors, at baseline, of your going on to accumulate this neocortical tau pathology that really does the damage in Alzheimer's. And what we found was that applying this method that we developed to identify this transentorhinal region, and measuring tau there, in combination with amyloid beta, did the best job of predicting who would go on to accumulate tau in additional brain regions onto your followup. So this really has implications for ongoing work in clinical trials, for these drugs that we're trying to treat Alzheimer's; we really want to get in at the earliest stages in order to hopefully prevent the catastrophic tau spread before it really becomes a problem for people.
Katie - Do you think this technique is about applying this to research methods, like you said, or do you think it could be used to actually diagnose people really early on?
Justin - At this stage our work is sort of translational in between the basic science, because there's still a lot that we don't know about Alzheimer's, its natural history and progression. But yeah, we're hopeful that in the long run this will be a very useful diagnostic tool for neurologists in the clinic; and hopefully catching it early on and being able to provide effective treatment strategies.

54:10 - QotW: Would a heavier Earth keep us from space?
QotW: Would a heavier Earth keep us from space?
Martin Khechara took on Stephen's question...
Martin - Since earliest history we have looked up and wondered what lay beyond the confines of Earth and marvelled at the stars and space. It wasn’t until 1961 when we managed to send the first person into space, when the Russian cosmonaut Yuri Gagarin orbited Earth on the Vostok spacecraft - closely followed by the American Alan Shepard Jr a month later. Would they have even made it up there if the Earth was a bit bigger? We asked Matteo Ceriotti, space systems engineer at the University of Glasgow.
Matteo - The answer is…it depends! A more massive Earth might not render rockets completely incapable, but it will certainly be harder to launch a given mass into orbit. Because a more massive Earth can pull harder on anything trying to leave.
Martin - And once the rocket reaches orbit, it needs to be orbiting at a certain speed to avoid falling back in.
Matteo - I considered an orbit such as the one of the International Space Station. To move 1 kg of mass from being stationary on the surface of the Earth, into orbit - which for the International Space Station is 420km above the surface - approximately 33 million Joules of energy are needed.
Martin - Since the first space rockets, this energy has almost entirely come from what is a fairly crude but controlled explosion, where hydrogen and oxygen are reacted to give upwards thrust. And all the energy in the fuel doesn’t go into pushing up the rocket, there’s a lot of light, heat and sound that come out as well. But 33 million Joules, theoretically, takes you up to the orbit of the space station. What if you’re launching off a much heavier planet?
Matteo - Let’s now imagine a new Earth which has twice the mass of our Earth, but is the same volume. In this case, the energy needed is approximately doubled (66 million Joules per kg).
Martin - Plus more and more energy needs more and more fuel. Eventually you couldn’t make a rocket big enough to hold it all! So ultimately, a heavier planet would make reaching orbit harder. Although - as Bored Chemist pointed out on our forum - if Earth was big enough, it would turn into a black hole. That would stop any rocket.










Comments
Add a comment