This week we'll need you to fasten your seatbelts because we're taking a trip into the future of fuels. We're asking if biofuels are really that brilliant and finding out how one lab is attempting to reinvent diesel. Plus, new research that could help unclog arteries and the data storage solution that operates at the scale of individual atoms.
In this episode
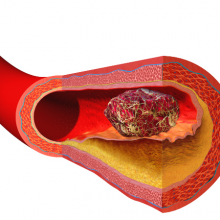
00:59 - Unblocking heart disease
Unblocking heart disease
with Professor Nicholas Leeper, Stanford University
Heart disease and strokes are the leading cause of death worldwide. They occur 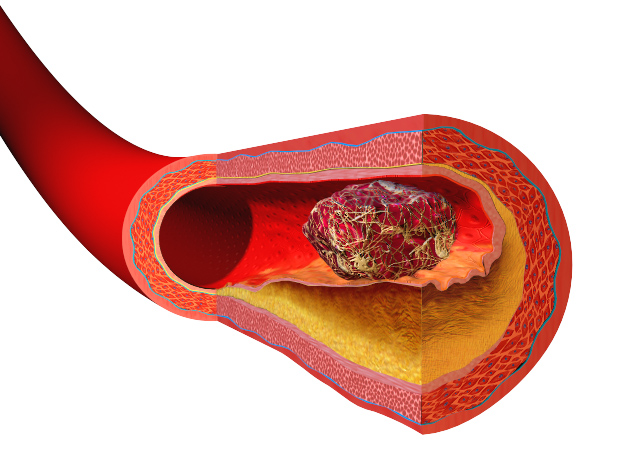 when blood vessels become furred up by fatty deposits called atheroma, which also contains dead or "apoptotic" cell debris, which the body seems to struggle to remove. Now Stanford scientist Nick Leeper thinks he knows why, and may also have got to the heart of solving the problem as he explained to Chris Smith...
when blood vessels become furred up by fatty deposits called atheroma, which also contains dead or "apoptotic" cell debris, which the body seems to struggle to remove. Now Stanford scientist Nick Leeper thinks he knows why, and may also have got to the heart of solving the problem as he explained to Chris Smith...
Nick - Our body will turn over as many as 100 billion cells per day, everyday. That is a process known as efferocytosis, and efferocytosis is Greek which means carry 'the dead to the grave,' and it essentially refers to the process by which a dying cell is recognised for clearance by a phagocytic cell like a macrophage. It's quite interesting because this process of efferocytosis is highly efficient and, actually, if you look very carefully throughout the body, it's nearly impossible to find any evidence of dying or apoptotic cells in healthy tissue.
The one exception to that is within the diseased blood vessel where there is really an amazing accumulation of these cells and other necrotic debris. And previously, we didn't quite understand why these cells were accumulating and not being cleared by the macrophages and other cells that seem to be so efficient at clearing cells elsewhere in the human body.
Chris - So if one looks around the body, every day the body is turning over, renewing, getting rid of dead cells and making new ones, and there's no evidence of the debris left behind. But for some reason, it doesn't get cleared away properly in the walls of blood vessels and this is what leads to the progressive narrowing of blood vessels and, therefore, their ultimate blockage?
Nick - That's exactly right. And previously our laboratory had decided to pursue the top GWAS locus for cardiovascular disease (GWAS stands for Genome Wide Association Studies). And this is work that was made possible by the humane genome project, where investigators could look for the first time across the full genome to identify hotspots on the chromosomes that are associated with risk for heart attack and stroke. We found that the top inherited locus for cardiovascular disease was associated with a novel pathway. People who are born with the genetic variance seem to have reduced expression of a so-called 'eat me' ligand, and this is a molecule that is expressed on the surface of a dying cell that makes it edible and appealing to these phagocytic cells like the macrophage. And we began to wonder if this was the driver of the accumulation of this debris in the growing atherosclerlotic lesion.
Chris - If that's true, why are cells in the rest of their body nonetheless cleared up efficiently then, if they're less digestible?
Nick - That's a great question. Investigators have long known that there is the presence of an inflammatory molecule called TNF-alpha that seems to accumulate and be responsible for the growth of the diseased vascular plaque. Now in the current study we've found that TNF-alpha seems to drive the expression of a don't eat me molecule. This molecule renders a cell inedible and so we think that in patients with developing aphroscotic plaque, local inflammation is driving expression of this antiphagocytic molecule that's rendering the cells inedible and unable to be cleared.
Chris - You're suggesting that blood vessels, when they're injured or damaged by disease, release inflammatory chemicals, including this chemical TNF-alpha, and that then turns on a don't eat me signal in the cells that are accumulating there, so they're not cleared and that leads to the progression and build up of cells and cell debris in the walls of arteries, narrowing them?
Nick - That's exactly right. And now we understand that this TNF-alpha promotes this don't eat me signal and this seems to make the cells inedible. It's almost like it's providing a cloaking device for these cells so that when they die they are invisible and unable to be cleared.
Chris - And if you take animals that are predestined to get furred up arteries and you block these signals in those animals, do they have lower levels of arterial clogging?
Nick - Exactly. What we found was, when we treated our mice who had very high cholesterols and were prone to developing atherosclerosis with antibodies that blocked this don't eat me signal, we found a profound improvement in cardiovascular disease.
Chris - You do think then that if you intervene in this way in a person who has established heart disease, you might be able to chemically unclog arteries?
Nick - Well that's our goal! Obviously our mouse findings are preliminary and need to be confirmed in human studies, but the extrapolation of our results would suggest that as we block the don't eat me signal, that we would be able to inhibit progression of atherosclerosis and perhaps even be able to melt away plaque by shrinking the necrotic core.

06:02 - Super small data storage solution
Super small data storage solution
with Dr Sander Otte, Delft University of Technology
From films to food ordering, our world is increasingly held in the digital sphere,  this change in behaviour has led to a vast increase in data and that data has to be stored. We've mentioned before on the programme how big data means big warehouses full of the hard drives, energy supplies and the aircon needed to keep them cool.
this change in behaviour has led to a vast increase in data and that data has to be stored. We've mentioned before on the programme how big data means big warehouses full of the hard drives, energy supplies and the aircon needed to keep them cool.
But now researchers at Delft University of Technology, in the Netherlands, have come up with a new way to store data at thousands of times the density... by using individual atoms. Claire Armstrong spoke with Sander Otte to hear how...
Sander - What we've been able to do is to essentially make a hard disk where you can store data in the forms of 0s and 1s, just like you do on a regular hard disk, except that here the bytes are all encoded by a single atom. There is just one atom for each byte and then the position of that atom tells you whether the byte is a 0 or a 1. You can really compare it to I think it's called an abacus... one of those old fashioned calculators where you move beads around...
Claire - Yes.
Sander - Here you are moving the atoms around. And that's exactly what you do and that's how we store the data.
Claire - Oh, OK. That's quite a nice analogy actually - an abacus. And how are you actually doing this - what kind of atoms are you using?
Sander - We are using chlorine atoms on a copper surface and that was actually the big discovery here. We found that these chlorine atoms on this crystalline copper surface, which is perfectly flat, also forms a very smooth regular grid of atoms. And then in that grid of atoms, we put on, just too few chlorine atoms essentially and, as a result, there were some atoms missing so you have holes in the surface. Then it really looks like a sliding puzzle where one piece is missing and you can slide a piece towards a hole and that's how you can move things around.
Claire - OK, wow!. So you're really controlling things here at the atomic level?
Sander - Yep.
Claire - How do you move an atom?
Sander - So we this work with a so-called tunneling microscope (an STM). That technique has been around since the 1980s. It consists of a very sharp needle that scans over the surface and it kind of looks like a record player, and that needle is so sharp that it can feel the individual atoms on the surface. So it scans over the surface line by line and then if you put all of those height profiles together, you get your picture of where the atoms are. Once the picture is finished you can then take your STM back to whichever atom you would like to move and then use that same needle to move it around. There's a few different techniques to do this, so either you can use the atom to drag them or, in some cases, you can even pick up the atom and put them down. But, in this particular study, we found we just needed to bring down the tip and, as a result, we inject a current basically in the atom that we want move and that current is in the order of a microamp, which sounds like a very small number but it's an enormous amount of current for that single atom, and that causes the atom locally to heat up a bit and that gives it enough energy to make the jump to the neighbouring empty site where it then goes to.
Claire - OK, so by using this very sharp needle and the current you can push the atoms around and, by pushing them to a different positions, that means you're storing different types of data. How fast can you read and write to this new hard drive?
Sander - Right. So there we come to the slightly less good news of this whole achievement, the read and write speed is still very slow. We actually didn't optimise to get to the fastest read or write speed yet so that in order to read the kilobyte we would need an hour, in order to re-write it we would need three hours. So that is almost ridiculous when we compare it to speeds that we normally have on our hard drive.
However, the dynamics of these atoms are ultrafast right. So, in principle, there is no limitation why this could not be sped up to let's say a megabyte per second as we see on a hard drive. However, I do recognise that there is quite a lot of technological barriers to be taken here, so it will definitely be a long time before we get there.
Data storage, to my opinion, is just an example here. It's a demonstration of what we are able to do but I think there is a much more profound message here which is, we have been able to manipulate the world on a scale that was just impossible before. So we humans have now been able to build things on a scale of individual atoms and, if you think about it, that would eventually enable us to perhaps even tinker with how materials work and what kind of properties materials have if we could really choose ourselves where the atoms go, we could build all kinds of circuits maybe on the atomic scale.
I don't know what the possibilities will be in the future, but I am sure that it will be way cooler than just data storage. Data storage is just a bit of a lame example in that sense and by lack of any imagination, it's the best we can show but there will be much more than that.

11:12 - Myth: NASA invented Teflon and Velcro
Myth: NASA invented Teflon and Velcro
with Dr Kat Arney, The Naked Scientists
In this week's Mythconception, Kat joined the Space Race in search of the  truth...
truth...
Kat - As British astronaut Tim Peake settles back into life here in earth, he'll probably be using several objects and materials that will be familiar to him from his mission, which were invented by NASA for their space missions. Maybe he's strapping on trainers with Velcro fastenings to go for a run, or frying up some breakfast in a Teflon-coated non-stick pan. Well, whatever he's doing, the real story is that although it's widely thought that Velcro and Teflon were invented by NASA for use in the space programme - they actually weren't.
Let's start with Velcro. It's true that the 1960s Apollo missions probably did more to popularise this useful material than anything else at the time - using it to secure items in spaceships under zero gravity, as well as fastening suits, sample collection bags and much more in the famous 1968 moon mission. But Velcro was actually patented in 1955, after being invented by a Swiss engineer named George de Mestral in the late 1940s, long before the space programme got going. He was out walking his dog one day and noticed that the poor creature was getting little seed pods, or burrs, caught in its fur. Looking more closely, he saw that the pods were covered in tiny hooks, which were locking into hairy loops in the furry coat of his pet but could be pulled off relatively easily. De Mestral then set about perfecting a two-component fastener system, with one part covered in tiny hooks and the other covered with soft loops. He called it Velcro - short for "velours crochet", the French for 'velvet hook' - and it soon became widely used for all kinds of domestic, industrial and military purposes, including heading out into space.
Then what about Teflon? Also widely used by NASA and other space agencies in heat shields, space suits and other structures and equipment, it's also a commonly held belief that NASA invented the stuff too. But, like Velcro, its invention predates the space race. Discovered by accident by DuPont Company scientist Roy Plunkett in 1938 while he was researching chemicals called chlorofluorocarbons, Teflon was first made when Plunkett stored some canisters of tetrafluorethylene gas at low temperatures to use for a chemical reaction at a later date. When the time came for his experiment, Plunkett was baffled to discover that the containers were no longer full of gas, but instead contained a white powder with some very unusual properties - it was resistant to heat, didn't react with other chemicals, and was also very slippery. Unexpectedly, the molecules of tetrafluorethylene has joined together - or polymerised - to create the world's first Teflon. And while it's tempting to believe that the stuff coating your non-stick frying pan has its origins in rocket science, it's sadly not true.
However, there are many useful devices and technologies that were invented by NASA that have made their way back down here to earth. For example, pressure suits based on astronaut's anti-gravity G-suits are now being used to treat women who suffer from severe bleeding after giving birth - a problem that claims around 70,000 women's lives every year, many of them in the world's poorest countries.And the miniaturised cameras developed by NASA's Jet Propulsion Lab in the 1990s, known as CMOS sensors, are used in most of the world's camera phones today. So while you're busy Snap-chatting, Instagramming or chasing Pokemon round the place, spare a moment to be grateful for this truly space-age technology.

15:08 - Can British science survive Brexit?
Can British science survive Brexit?
with Venki Ramikrishnan, The Royal Society
In June 2016, the UK voted to leave the EU. Now the dust has settled, conversations are starting up around what this means for things like UK research. This week, a number of the country's leading scientific institutions have issued a joint statement setting out what what they regard as the priorities needed to ensure that the UK remains a world leader in the scientific arena. Chris Smith went to see Venki Ramakrishnan, winner of the 2009 Nobel Prize for Chemistry and current president of the Royal Society, which is one of the entities behind this week's statement...
Venki - Before Brexit, the science community received quite a lot of funding from the EU; it amounts to about 10% of academic research funding and it turns out that British scientists are actually very successful. So we get back more from the EU than proportionally we would have put in to the EU science programmes. That's not true of the overall contribution to the EU budget but I'm simply talking about the science part.
Chris - And that 10%, to give people some perspective, how much is that worth in monetary terms?
Venki - I think it's about 6 billion or so!
Chris - That's a big slug of money!
Venki - It's a big chunk of money but it is 10%. But what I would say is that more than the money, the EU allowed British scientists to be part of large networks, large scale collaborations, big science that couldn't be done in any one country or by a small team. And it's these networks and collaborations that we think are even more important than the funding itself.
Chris - So there are two challenges immediately to surmount here. One is that we do potentially face a 10% drop in funding in science in the short term at least in the UK, but the other is access to these wider European networks. Why would they necessarily be closed to Britain then?
Venki - Well, if you want to be a full participant and you want to be part of the single market, then they'd require full mobility. And Switzerland, for instance, had a referendum in which they didn't want to include Croatia in the free mobility into Switzerland and the EU responded very promptly by cutting them off from many of the EU science programmes. Now those programmes have been temporarily restored while Switzerland and the EU are negotiating but, my view is that given what has happened since with Brexit, the EU will not be predisposed to making things easy.
Chris - I was going to say are we not looking at this in a slightly oneway street view in the sense that Britain is a major player scientifically in the World, and especially in Europe. Therefore it's also going to hurt Europe to ostracize the UK, isn't it?
Venki - Precisely, and so so what I'm hoping is that our EU partners put pressure on their governments, as much as we try to persuade our own, so that some common sense agreement can be reached so that we can continue to be part of an EU wide scientific activity, which will benefit both of the rest of the EU and Britain.
Chris - If one looks at the big scale projects in Europe; things like the Large Hadron Collider, which has investment from multiple entities across the European Union, actually that's not under the direct jurisdiction of the EU, is it? So actually, they couldn't turn round to us and say - well you're not in the club you can't now be part of the LHC?
Venki - You're absolutely right! So things like CERN and the European Space Agency, they're all outside of the EU in the sense that they're autonomous organisations; they're funded by member states and, in fact, they have membership outside Europe, But the reality is that the EU actually funds programmes for scientists to get together to plan some of the use of these large facilities and so on, and it's these participatory networks that we could be shut out of.
Chris - You're the President of the Royal Society - have you had Downing Street or equivalent on the phone to you saying - what do we do about this?
Venki - We have had conversations. I've had a letter that was copied to me from the Prime Minister's Office. I've had discussions with various people and there does seem to be a general agreement that science is very important to the UK and they intend to try and make it work. So I would say I am cautiously optimistic at this stage that this government is very well aware of the importance of science and innovation, and, in fact, in a post Brexit environment, I would argue that if we want to thrive as an economy it becomes even more important because we don't necessarily have a guaranteed arrangement as we would have had within the EU. And so, I'm hopeful that the government will act in a way that tries to preserve these interactions, tries to preserve our ability to recruit some of the best talent, both from Europe and around the World, and also make sure that the overall science funding is preserved. Because after all, if the argument that the Brexit people made that we would save money by leaving the EU, well some of that money could go back into science.

21:05 - A sweet tale of cooperation
A sweet tale of cooperation
with Dr Claire Spottiswoode, the University of Cambridge and the University of Cape Town
The honey guide is a wild bird native to Africa and Asia. Incredibly, these wild birds naturally respond to calls from human honey hunters and lead them to bees' nests. The people subdue the bees and get the honey while the birds take the leftover wax. Connie Orbach spoke with University of Cape Town researcher Claire Spottiswoode who's been out to Mozambique to study the phenomenon...
naturally respond to calls from human honey hunters and lead them to bees' nests. The people subdue the bees and get the honey while the birds take the leftover wax. Connie Orbach spoke with University of Cape Town researcher Claire Spottiswoode who's been out to Mozambique to study the phenomenon...
Claire - So that was the sound of a Ziwere honey hunter in the Niassa National Reserve in northern Mozambique and he was summoning a bird from the bush to help him... 'brrr-hm'. And then you heard the chattering of a small brown bird: the greater honeyguide, which is an intriguingly odd species. Its favourite food is wax and it cooperates with our own species to get it.
So honeyguides love wax and they know where bees nests are located but there aren't much good at doing battle with the bees to get it out of their nests. Whereas human, by contrast, as everyone knows, love honey and are experts at getting into these nests with the help of fire and tools. So the bird, through its calls and behaviour, indicates the location of these nests to honey hunters and, in turn, humans use their sophisticated tools and fire to create smoke to subdue the bees to open the nest and expose the wax for the honeyguides, and the honey for the humans.
Connie - That's amazing. And so these honeyguides are wild?
Claire - Yes, they're not domesticated, or tamed, or taught, or coerced in any way.
Connie - And you've been studying this - what have you been looking into?
Claire - Well, our aim was to distinguish whether honeyguides could specifically recognise and respond appropriately to signals directed at them by honey hunters. So we recorded a number of honey hunters making that special sound 'brrr-hm'. And then we recorded the same individual honey hunters giving arbitrary noises that we asked them to call out, and these were either their names or words in the local Yao language for honey and honeyguide.
So if honeyguides specifically recognise the information content of the "brr-hm' sound, then they should be more likely to respond to a human making that sound versus other arbitrary human sounds that don't carry the same specific meaning.
Connie - And you found that there was a relationship between that specific sound, that I'm not going to do because I'd make a fool of myself, as opposed to the control sounds you used?
Claire - Yes, when we made the 'brrr-hm' call, we were twice as likely to be guided by honeyguides compared to the control sounds. Not only that, when we made these arbitrary human noises or arbitrary animal noises, honeyguides often lost interest and stopped guiding us. So the upshot of all that was the overall chance of being shown a bees nest by a honeyguide was actually more than tripled from around a 16% success rate to a 54% success rate.
Connie - So this need for a specific word - does that mean there's some sort of language going on? Are the honey hunters speaking to the honeyguides?
Claire - Certainly from a layman's perspective, i.e. my perspective as someone who's not a linguist, it certainly does seem like a kind of proto language where bird species understand the specific signals that they're giving to one another.
Connie - And so what do you think led to this interaction? Is it just a behavioural adaption that the birds learned through their lifetime or do you think there's more to it than that?
Claire - Well honeyguides, they're also brood parasites, so like cuckoos, they lay their eggs in other birds' nests and foist the cost of raising their young onto other species. So one intriguing consequence of this brood parasitic start to life is that honeyguides don't have the opportunity to learn from their own parents, so our best guess is that their propensity to guide humans is probably genetically inherited.
Having said that, we've now seen a remarkable local adaptation to a specific signal used by a particular human population co-existing with honeyguides and again, this is only a guess, but our best guess is that those local refinements are probably acquired through learning rather than being genetically inherited.
Connie - So, if this is an evolutionary adaptation, that gives me the idea that this should have been going on for a very, very long time. How long have the honey hunters been cooperating with birds and where did their language come from?
Claire - So to answer your first question about the age of the human-honeyguide association in general - well it's hard to say but it's probably very ancient indeed. But you also asked me about the origin of the particular call that humans use to cooperate with honeyguides and I've asked this question to the Yao honey hunters in Mozambique on many occasions, and they always say to me well we do it because our dads did it, they learnt it from their dads and we really have no idea. It's just something that we do. Intriguingly they also say "no-one will disturb this thing." What it emphasises is that there's no incentive for any individual honeyguide to use any other signal because, of course, they'll end up finding less honey. So there's a sort of positive reinforcement that maintains that language in the population.

26:38 - Taking a look under the hood
Taking a look under the hood
with Philip Garsed, The University of Cambridge
What actually goes on under the bonnet of a vehicle powered by a combustion  engine burning petrol or diesel? Cambridge University's Philip Garsed got moving with Naked Scientist Connie Orbach...
engine burning petrol or diesel? Cambridge University's Philip Garsed got moving with Naked Scientist Connie Orbach...
Connie - Like many people I've got a car and I actually used to drive a lot for my old job, but despite my absolute reliance on gas guzzlers, I've never actually known how they work. To change that embarrassing fact, I asked Cambridge University's Philip Garsed to take me through the basics...
Philip - It's quite simple in many respects. All a petrol engine really is is a series of cylinders, and inside the cylinders are things called pistons, which are basically big bits of metal which kind of seal nicely to the cylinder and can move up and down, and these pistons move up and down, because of the fact that the engine is moving. The engine works by having the piston move up and down in a series of strokes.
So in the first stage you need to get the fuel and the air into the engine, so the piston moves down and it sucks in a mixture of air and fuel into the cylinder, because it's sort of moving down and pulling in this mixture.
At that point a valve closes, the piston continues its movement and starts to move back up again, but because now this mixture is stuck inside the cylinder, it gets compressed really hard and you end up with all this very flammable air and fuel in a very, very small space.
At the point where everything is most compressed we ignite a little spark and that causes this fuel and air mixture to burn very, very quickly, and as it burns it gets hot and that forces down on this piston. That's where we get this mechanical energy out of the fuel - so that's stroke number three.
The final thing that happens is we open a second valve and as the piston continues its journey and goes back up again, it forces all that hot air and gas out of the cylinder and then that goes to the exhaust pipe.
So there's four strokes : bringing in the fuel and air, compressing the fuel and air, igniting it and burning it, and then exhausting it.
Connie - OK. We have this clever four stroke piston but only one of the strokes is producing your power to drive the car forward. So even if it's working at superspeed, that sounds like a pretty clunky journey to me...
Philip - Yes, if you just had one cylinder it would be very, very bumpy. You'd get a periodic big shove of mechanical energy and not much the rest of the time, which is why in real life, in a car you have several cylinders. It depends on the size of the car; you can have 4, 8, 16. You arrange it so that they are doing all of these parts of the cycle at different times and they all go slightly out of sequence with each other, and that gives us much better, much smoother power output.
Connie - Philip mentioned that the movement of the pistons is provided by the movement of the car but, unless I'm very, very confused, most cars start from a stationary position...
Philip - The engine can only keep moving by already being moving, which is why we need things like starter motors. When you're starting your car you turn the ignition and you hear it sort of whirring up and that's an electric motor that's causing the pistons to move up and down in the right way, so that we can at exactly the right time bring in the fuel, burn it, and get the thing going. In the olden days you had to do that by hand and it was a pretty hard job turning the engine over to get it to start.
Connie - So that's why when my battery's flat I can't start my engine. It's because this initial electrical impulse isn't there to get the thing moving?
Philip - Yes. If you've got no battery, the motor can't turn and your engine is just sitting there. But, if your battery is flat, one thing you can do is push the car along until it's going fast enough, and sometimes, if you get your timing exactly right, you can get it to start that way.
Connie - Wow, I always through a push start was just some kind of...magic? But, my naeivity aside, petrol isn't the only fuel available - what's different about diesel?
Philip - In some ways it's quite similar. A lot of diesel engines have this four stroke idea but what you do with the fuel is a little bit different. Because diesel engines tend to use a heavier fuel it means there's a bit more energy in the fuel itself (from a certain amount of it) but it's a bit harder to get that energy out.
And so, instead of bringing in fuel and air when you're sucking things into the engine, you just bring in air. Once you have that air stuck inside the cylinder, you then compress it really, really hard (far more than in a petrol engine) and actually, if you compress air it tends to get very, very hot. And so, when you've got to the point where the air is at its most compressed, then you inject the fuel straight into the cylinder and it almost immediately vapourises and then, because it's so hot there it burns, and it burns nicely and consistently and that's the point at which it forces the piston back down again.
So the difference is in how we put the fuel into the cylinder after that it's kind of quite similar.
Connie - So what is it about diesel and petrol (the fuels themselves) that means you need these two different systems?
Philip - Well petrol is basically a lighter kind of fuel. It evaporates more easily whereas diesel is much heavier but stores more energy. So petrol engines are good because they have fewer pollutants, they tend to be quieter. Diesel engines you can get much more energy out of them, so they're good for really big, heavy duty engines.
Connie - So diesels, though we have them in cars, you're more likely to see them in, what, lorries and trains and things like this?
Philip - Yes. It would be very unusual to see a petrol train or lorry; mostly they use very, very big diesel engines

32:44 - Reinventing diesel
Reinventing diesel
with Professor Mani Sarathy, King Abdullah University of Science and Technology (KAUST)
Diesel and petrol engines work in different ways, which means that they need totally different forms of fuel. But here's the problem. Engineers and petro-chemists are predicting that, within the next decade or two petrol will no longer exist. We'll all either be behind the wheel of an electric car, or driving large transporters powered by diesel.
So what happens to the 20% of crude oil chemicals that currently go into making petrol? You can't just add them to diesel fuel because the present generation of engines can't burn them. So researchers at the King Abdullah University of Science and Technology in Saudi Arabia are developing new forms of fuel - and new diesel engine designs - that can. Combustion chemist Mani Sarathy showed Chris Smith around...
Mani - So we're working closely with industry here in Saudi Arabia, as well as around the world, to look at how we can maximise the usage of fuels that are generated from a barrel of crude oil. Right now, crude oil is used to make gasoline fuel for light duty vehicles and diesel fuel for heavy duty vehicles
In the future we see that light duty vehicles will be running probably on electricity. As a result, there's going to be an oversupply of gasoline. And our goal, as a research centre, is to make sure that fuels like gasoline (we call them naftas) can be utilised in diesel, heavy duty applications.
At the refinery side we actually want to minimise the amount of refining steps because the less refining you do, the less energy intensive it is. But less refined fuels will also not work in a conventional diesel engine so, at the same time, we are trying to promote a new type of diesel engine application - we call it a partially pre-mixed compression ignition engine. The diesel engine operates in a slightly different mode so that it can burn a larger variety of fuels.
The first step is we need to understand what the engine wants. For that to happen we really need to understand the combustion process in the engine. So these new types of pre-mixed charge, compression, ignition engines require fuels to autoignite with some specific properties, and we call that autoignition property the ignition delay time. So, if we know this parameter, then we can say how can we formulate the fuel to provide the ignition delay time that the engine wants?
Chris - Is that what you're doing in here?
Mani - Yes. So my colleague operates a shock tube lab, looking at how fuels would behave, different types of fuels at different temperatures, different pressures, and varying mixing fractions of fuel and air.
Chris - Shall we take a look?
Mani - Sounds good...
Chris - This is a big room and there are a sequence of very, very large stainless steel pipes. They're what - 6 inches in diameter pipes stretching the length of the room. What are they - what do you do with them?
Mani - The pipes are roughly separated in half. Four metres is what we call the drivers section of the shock tube; four metres is the driven section, and these two sections of pipe are separated by a diaphragm. This is the diaphragm; it's made of aluminium and on the diaphragm there's a score. The score is to facilitate the bursting of the diaphragm.
Then on the driver section an inert gas is injected up to a very high pressure and then we apply a slight prick to the diaphragm. As the diaphragm bursts, a shock wave will be accelerated down the second half of the tube. In the second half of the tube there's a mixture of fuel and air. As the shock wave travels down it compresses the test gases and then the shock wave hits the end of this tube and reflects back, and as it reflects back that reflected shock further compresses this fuel/air mixture.
There is a series of pressure sensors to determine the shock speed; also to determine what is the pressure of the test gas section.
Chris - What about the chemical composition of what's going on in there - can you see that going on too?
Mani - Absolutely! So, as the reflected shock goes back and heats up the fuel/air mixture, the fuel starts to oxidise and undergo various reactions. These can be captured by a number of diagnostic techniques based on lasers.
Chris - And by shining laser light in, are you relying on the fact that certain colours or wavelengths of laser light will be absorbed by certain chemicals that are being produced by the reactions?
Mani - That's pretty much what happens. Different molecules are going to absorb different wavelengths of light. So we have a spectral database which tells us: CO2, hydrogen peroxide, carbon monoxide, different types of hydrocarbons which are the intermediates of a combustion process. Then you have detectors tuned to detect light coming out at the other end. And then it's a simple relationship (what we call Bio-Lambert's law), which relates the absorption with the concentration of the gas in the test section and we can monitor this over about a one microsecond.
Chris - The thing is a big 6 inch caliber stainless tube is not however an engine...
Mani - It's not replaceable for the real thing but what it's really good for is making sure our models work well. Now we have accurate chemical kinetic models for our fuels. We can take the fuel formations which we have tested here and we can go and test them in our engine facility...
Chris - Well it certainly smells like an engine room in here - that beautiful oily smell that people like fiddling cars will be acquainted with. We've got two big engines in here and what's the difference between the two?
Mani - One is primarily used for a gasolene engine, so it's a spark ignition engine and the other is meant mainly for diesel engine research as a compression ignition engine.
Chris - And they're all rigged up with a whole panolply of different sensors so that you can monitor the conditions going on?
Mani - Yes. So just like in the shock tube lab we were very concerned with the temperature, the pressure, and the mixture, and how that affects the ignition process. The same way in an engine you've got to control these three parameters: we have compressors to control the pressure intake, we sometimes have heating jackets to control the temperature of the intake stream, we can vary the engine speed to determine the temperature inside the cylinder, the pressure inside the cylinder and, by varying the amount of fuel injected, we vary the mixture fraction inside the cylinder, the mixture stratification. We also measure the emissions that you would be concerned about coming from your engine.
Chris - And then what - do you go back to the automotive manufacturers and say right, look, we know what the fuels can do, this is what you need the engines to do to accommodate these fuels?
Mani - That's exactly right. And there's also an engine research community that's helping to develop new types of engine combustion modes and the research community has developed these engines and said these are the types of engines that are more efficient, cleaner burning, these are the combustion engines of the future. In order for industry to adopt them, industry needs to be comfortable, not only that they can make these engines, but they're also going to have the fuel that these engines require.

39:52 - Brilliant biofuels?
Brilliant biofuels?
with Dr Christopher Chuck, The University of Bath
We could keep refining the burning process of crude oil to make the system as  efficient as possible, but why do we have to use fossil fuels at all? Biofuels have been around for the last few decades and they can be used in pre existing engines. But if they're so great, why do they only make up a tiny fraction of the fuel we put in our tanks? Chris Chuck is a researcher from the University of Bath and he took Kat Arney through the basics of biofuels...
efficient as possible, but why do we have to use fossil fuels at all? Biofuels have been around for the last few decades and they can be used in pre existing engines. But if they're so great, why do they only make up a tiny fraction of the fuel we put in our tanks? Chris Chuck is a researcher from the University of Bath and he took Kat Arney through the basics of biofuels...
Chris - Well simply, a biofuel is just a liquid fuel where most of the molecule, if not all of the molecule, is derived from a source of biomass. Now this biomass could be vegetable oil, sugars, plants, grass, waste food, any type of plant biomass.
Kat - When we hear about people running their cars off old chip fat - that's a biofuel?
Chris - That's absolutely a biofuel. Yes, that was one of the early ones.
Kat - In places like Brazil, as well, they run cars of ethanol and that's derived from plants as well?
Chris - Yes, so in Brazil they're using sugar cane. They're growing sugar cane in the vast areas of lands that are suitable for that and turning it into ethanol; it's one of the most successful biofuels on the market.
Kat - So we've got all these different biofuels from different sources - what are some of these sources? You've talked briefly about some of them and are they all the same, are they all just equivalently good or bad?
Chris - There's a great diversity of biofuels either on the market or being developed at the moment. Some are to fit the role of petrol, diesel, or aviation fuel, which all have different properties. In other kinds, it's where you actually derive them from.
So we really have two definitions of a biofuel whether it's a first generation biofuel, made from food matter or it's grown on land needed for food production. Or second generation biofuels where the biomass doesn't compete with food and this is a much, much bigger resource. So we can talk about agricultural residues, forestry waste, waste food, even algae and seaweed that's produced biofuels from this. Now because they're so diverse these different types of biomass, we have to use lots of different chemical and biological processes to make the fuels from them.
Kat - And does this mean that, actually, they may be not as efficient as just drilling the stuff out of the ground?
Chris - Well yes, it very much depends on what land area you've got to grow them on, what's in place in terms of the infrastructure to make them, for example, bioethanol from sugarcane has been very well developed in Brazil and is actually a lot of the time, depending on the cost of a barrel of oil, cheaper than petrol there on the market as well. But most of what's limiting biofuel manufacture at the moment is actually the cost implications.
Kat - There is this question though because it's being estimated that the fossil fuels that we have for car fuels and that kind of thing, we've probably got a little bit more than we thought we had. And if the idea is that we'll eventually all be running on electric cars, are biofuels really more like just a stopgap? Do we need to put time, and effort, and money into making them a replacement for fossil fuels?
Chris - Well this is quite a common misconception. Now a lot of people have been looking at what our energy mix will look like in 2050 or even 2100. Everyone disagrees entirely on the numbers and what it can be made up of. But one thing all of these major models have suggested is that we need to increase the amount of biofuels being used and, if you take a rough average, it's approximately 50% of our transport fuel will need to come from biofuels by 2050. And our electricity networks and other forms of energy generation just can't meet that gap.
Kat - Obviously things like plants, you use carbon dioxide to grow; they're taking carbon dioxide out of the environment. Is that alteration of the carbon balance something we should be taking into account almost regardless of the cost?
Chris - Absolutely! It's very complex to work out whether a biofuel actually is sustainable, actually is reducing carbon emissions on using fossil fuels and, in the last few decades, we've been developing the tools made in life cycle assessment to be able to make those statements, to be able to understand that. And so we really need drivers to push the biofuels which are showing very big promise in reducing greenhouse gas emissions.
Kat - Because, I guess, they're taking the carbon dioxide in from the environment, you're still burning it and putting it back out, but that's almost like a closed loop, I suppose?
Chris - Absolutely, but then you have to work out how much energy are you putting in transporting the biomass, drying the biomass, using the fertilisers even to grow the crops. So you can have enormous energy inputs without really counting them unless you're careful. It's very important that you assess exactly how a biofuel is made from growing it in the field all the way to emissions when you've burnt it.
Kat - And just very briefly - are biofuels already out there? Could I be putting biofuels in my car or using biofuels in a way that maybe I don't realise?
Chris - Absolutely! About 5% of total transport fuel in the world is now biofuel but, ultimately, you are using them and you've probably used them today already. They're doped into fuels at a few percent just to get them into the infrastructure and so there's not a great choice that you can say - I'm going to use biofuel or not use biofuel. It's already a percentage in the fuel that we already use.

45:04 - Decreasing the pressure on NGVs
Decreasing the pressure on NGVs
with Dr David Fairen-Jimenez, The University of Cambridge
Biofuels aren't the only alternative to petrol and diesel. Natural gas is also an  established technology that we know works and, because it contains more hydrogen relative to carbon, the resulting emissions are cleaner. But there's still a catch, as Chris found out from Cambridge University's David Fairen-Jimenez...
established technology that we know works and, because it contains more hydrogen relative to carbon, the resulting emissions are cleaner. But there's still a catch, as Chris found out from Cambridge University's David Fairen-Jimenez...
David - So the main problem with using natural gas is that it's a gas not a liquid. So you need to use extremely high pressure in order to store this amount of energy you need for vehicles.
Chris - And why should high pressure be a problem?
David - Well it is related to safety first of all. So if you have a car accident. 200 bar is a really, really high pressure (it's 200 atmospheres of pressure), but it is also quite expensive so we need to use compressors.
Chris - Putting that another way then you're saying that to the store the stuff you need very high pressure cylinders, which they need to be built of strong stuff which weighs a lot, and then just packing the gas in is taking a lot more energy because you're shoving it in under pressure and that adds the cost up?
David - Yes, exactly. And also the main problem is refilling these tanks, it's going to be very difficult. So it's very easy to have just a couple of vehicles that are tending the tanks but if we assume that every single vehicle is using natural gas we will need petrol stations like we have now...
Chris - All equipped with very high pressure...
David - Yes, safety is going to be an extremely big problem and also economics as well.
Chris - And your answer?
David - And the answer is to use an alternative which is based on absorbed natural gas. So basically what we do is using like a porous material in order to be able to store this natural gas as a liquid inside the porosity.
Chris - These materials - they're call MOFs aren't they (metal organic frameworks) - they look like honeycomb I suppose at the nanoscale...
David - Exactly. So we use this nano material which has a microporosity which is on the size of the molecular size. So these really, really tiny holes we have in this material gives extremely high surface areas. We're talking sometimes up to 10,000 square meters in a single gram of the material.
Chris - Right, so you've got a material that's got lots of holes in it and the gas can go into those holes and stick to the sides, stick to the honeycomb I suppose, the surfaces?
David - We have extremely high interactions between the walls of the porosity in these materials and then these gas molecules are stored at much, much lower pressure.
Chris - Are you saying that the structure of the honeycomb, is such that it's got some kind of affinity, or it likes the gas and the gas likes it, so the gas will sort of stick itself on, and that means instead of having to ram the gas in really hard under pressure it just goes in there under much less pressure?
David - Yes, exactly. So the best example to see how the process works is say, when you are having a shower and you have all the vapour from the hot water. It's condensing on the mirror because it's a little bit colder than the rest of the room, so this is exactly the same phenomena.
Chris - You're pulling the gas out of the gas and onto the walls?
David - On the gas face, to the solid face.
Chris - And what materials are they? How do you make the gas stick to this material in this way?
David - Everything is about absorption technologies and about 20 years ago we started using these metal organic frameworks, so really new materials. And we can really tune the properties of these materials from an atomic point of view. So we can really select the building blocks that we have in these materials. We have like a 3D lego that we can create to play with this construct.
Chris - And if you can tweak the chemical formula for them, does that mean you can almost make like a molecular filter where you can chose the chemistry that's going to select for the gas you want, and you could just chuck it in a mixture of gases and it will pull out the one it wants and store it?
David - Yes. exactly. We can improve the absorption capacity of the material or we can improve the selectivity of the material. So we can tune these chemical properties or sometimes even the size, so we may have materials with very tiny porosity, very well defined. Some molecules will be able to got through this porosity, some others will not. This is going to be a molecular sieve.
Chris - And how much gas can one pack into a cylinder which you would fill with this porous material instead of just having an empty space then?
David - The Department of Energy for the United States, for example, they have the targets of storing the same amount of oil that you need to store at 250 bar but at 60 bar, so it's much, much lower pressure but needs to have exactly the same capacity.
Chris - It's still pretty high pressure though, isn't it - 60 atmosphere, 60 bar?
David - 60 bar is also really high but it is really nice in terms of the cost because you don't need to have two stage compressors, you only need to have one single stage compressor, which means that it's much easier to included into petrol stations at the end.
Chris - What's the uptake been like and is there interest from industry to actually deploy this?
David - There are a lot of companies that are very interested in this technology. The first one was BASF, a German company. They started synthesising these materials and you can buy them but they are very expensive right now because they are sold in a very, very low scale. And there are also a lot of spinouts coming out from universities.
Chris - Dare I ask when?
David - It's difficult to know when they are going to start using these materials. But it would be nice to see something in ten years time at least.

50:27 - Why does my stomach rumble when I'm hungry?
Why does my stomach rumble when I'm hungry?
Lucka Bibic asked Dr Rashini Raj from Medical Trinity Center in New York for an answer to this noisy question...
Rashini - Stomach rumbling or growling as we call it can be very common and quite normal in most cases. What's happening here is your digestive tract is a hollow tube that moves food and drink along. When you have a mixture of liquid, food, gas, moving along through the intestine, that can cause noises - sometimes quite loud.
Now when we're hungry our brain sends signals to our stomach to secrete juices (so that's a liquid). It also sends signals to our intestine to start contracting, moving those juices and air along and because this hollow tube is empty at this moment, the noises are more amplified and you might hear them more.
Lucka - Roger that Doc! What if these loud stomach noises come to the point that it's quite embarrassing?
Rashini - Now if you're hearing more noises than usual when you're hungry or any time during the day, and it's something different from your normal pattern, you may want to have this investigated with a doctor. Because, occasionally, increased bowel sounds as we call them are noises from your intestine, could be a sign that you're not digesting your food properly. If you're not digesting food properly, more gas can be released and then this can cause more noises being generated when the muscles of the intestinal wall are contracting and causing what we call peristalsis, which the motion of food and liquid being moved along the hollow tube of your digestive tract.
Lucka - So either feel the stomach or give your belly the room to process and break down your food properly. Is there any other way to quiet the beast?
Rashini - Certain foods can produce more gas which, in turn, can produce louder noises. Things like beans, cauliflower, cabbage, broccoli, foods or drinks that contain lactose, gluten or wheat for certain people. These may also cause more stomach rumbling so, if you're having an issue, you may want to try cutting out some of these foods and see if you notice a difference. Even something as simple as chewing gum causes you to swallow more air which then, in your intestine, can cause more noisy peristalsis.
Lucka - But no worries, there's always a silver lining to everything! Right Rashini?
Rashini - In general, stomach rumbling is generally nothing to worry about. You may be consuming a few more gassy foods than you should be and you might want to try cutting down on these if the stomach rumbling is really bothering you. But, for the most part, it's a normal, healthy part of digestion so just don't worry about it too much.
Lucka - Thanks Rashini. Next week we will be answering Tony's question:
If you could slice the Earth in half and leave the inner and outer core intact, like cutting an avocado - what would it look like from space? Kat - From the human stomach to the belly of the earth, if you think you know the answer to this one then send it in to chris at naked scientists dot com, tweet at naked scientists or get stuck in on our forum, naked scientists dot com forward slash forum.










Comments
Add a comment