This week we investigate green clean ups. Can nature's recyclers, bacteria and fungi, help us clean up man-made environmental problems from oil spills to mining slag heaps? Plus in the news, how the Gemini Planet Imager is helping astronomers 'see' exoplanets, why pregnant women are at a higher risk of a car crash and why don't octopuses get tied up in knots?
In this episode

00:55 - Car crash risk during pregnancy
Car crash risk during pregnancy
with Don Redelmeier, University of Toronto
Pregnancy is one of the most exciting, but potentially also one of the most dangerous times in a woman's life. But it's not just the threat to her health that comes from the physical demands of having a baby. It turns out that women in the middle parts of their pregnancies - the so-called second trimester - are much more likely to be involved in life-threatening car accidents. In fact, according to a new study looking at over half a million Canadian women, the risk of ending up seriously injured in hospital following a collision may be over 40% higher during pregnancy. Don Redelmeier, from the University of Toronto, led the study...
Don - Pregnant women often ask me the strangest questions about scuba diving, roller coasters, airline flights, and even grizzly bear attacks. They almost never ask me about the everyday risks. I was never once asked about road safety despite of being a much larger risk.
Chris - So, you were interested in addressing the fact that there is very, very little data actually looking at whether being pregnant does affect her own safety.
Don - Absolutely right. Standard prenatal care guidelines are virtually silent about the importance of road safety and that includes guidelines in the United Kingdom the United States, Australia, and Canada. We thought that that was sort of a major omission in the existing medical literature.
Chris - So, what did you do to try to fill that gap? How did you approach that?
Don - We identified every woman in Ontario, Canada who gave birth during a 5-year span and we tracked each person for any emergency department visit related to a road crash that occurred either before, during or after pregnancy. So, that amounts to a sample size of over half a million women and about 8,000 life-threatening motor vehicle crashes.
Chris - And that study design inevitably means that, because you've got a person who is pregnant at some points, and not pregnant at other points when they might be having a crash, you can directly compare their vehicle accident risk when they are and aren't pregnant?
Don - Exactly right, Chris. It's a huge strength of the study and that each individual serves as their own control. And thereby, we remove confounding from genetics or personality, education, lifestyle, or all sorts of other unmeasured factors that could contribute to roadway risks.
Chris - So, when you do the comparison and ask, is someone more likely to have a crash when they're pregnant compared with when they're not, what trends emerge?
Don - Our largest single finding was that we found that the second trimester of pregnancy led a to a 42% increase in the risk of a serious motor vehicle crash compared to the very same months for the very same person before they were pregnant.
Chris - Do you have any idea as to why you should see that profound escalation in risk and are the numbers big enough to support? Is that statistically and clinically significant finding?
Don - It's an extraordinarily strong finding from a statistical point of view. It's far beyond any explanation related to random chance. One possible theory is that the increase in risk related to a combination of fatigue, insomnia, anxiety, nausea, distraction, excitement, that are all parts of a normal pregnancy and yet, could contribute to driver error.
Chris - Why do you think there is that hotspot in the second trimester of pregnancy? Why not right at the beginning or people might think more obviously, at the end of pregnancy when people are really quite encumbered by - in some cases, a very big belly and some cases, with twins in there?
Don - I think that the extenuation in the middle trimester occurs because that's when women are usually feeling at their best. That's when they're trying to make up for lost time in a first trimester as well as get everything done possible before the baby comes. So that there's a lot of rushing around and there's also potentially a false sense of security in the middle months that needs individuals to potentially lower their guard.
Chris - And when you look at the sorts of accidents that the women are involved in, is there any sort of blame attributed there? Can you see whether they're no claim or it's that person's fault or it was the other person's fault? Who is causing these accidents?
Don - We don't do any at fault analysis. So, it might be that their pregnancy merely impairs a woman's ability to avoid the motor vehicle collision that was setup by somebody else.
Chris - It's interesting because people have looked at people's cognitive performance during pregnancy. They've even looked at people's driving performance all be it using a simulator during pregnancy and they haven't seen these trends or something that would produce such a big increase in risk, but you see. So, how do you account for that?
Don - The past literature about baby brain is really quite controversial, Chris. In community surveys, about 50% of women will describe intermittent episodes of forgetfulness where they misplaced their wallet or they forget about an important appointment. And yeah, most laboratory studies have not been able to replicate large changes in psychology performance. A limitation of those data though is that they're often based on small samples of individuals engaged in artificial tasks and sort of hypothetical risks. So that the strength of our study is, we look at real world driving over an enormous population without any selection bias.
Chris - Do you think that what you're seeing on the roads could be a proxy for a more general phenomenon? Do you think that there could be more general advice given to women in the second trimester of their pregnancy that they are more likely to have other sorts of lapses or accidents and therefore, they need to be more comprehensively careful?
Don - We looked at that pretty carefully in terms of involvement as pedestrians and we didn't find any increase in risk. We didn't find any increase in falls or poisonings, or burn incidents. So instead, the increase in risk is solely isolated to high speed traffic where a small lapse of attention, 1/10th of a second could lead to irreparable consequences.
07:44 - Predicting pre-term labour
Predicting pre-term labour
with Stephen Lye and Jan Heng, Mount Sinai Hospital in Toronto
In the UK, around seven in every 100 births is premature, happening before 37 weeks of pregnancy. Happily for mums and their babies, medical advances have brought big changes in survival and long-term outcomes for these tiny tots, but it's still difficult to tell whether a women having early contractions is  just having a false alarm, or is about to go into labour and needs treatments such as steroids to help the baby's premature lungs. Now Stephen Lye and Jan Heng at Mount Sinai Hospital in Toronto have developed a test that uses the levels of gene activity - that's messenger RNA or mRNA - to spot women who are about to deliver early, by looking for genes linked to inflammation, which is part of the natural birth process.
just having a false alarm, or is about to go into labour and needs treatments such as steroids to help the baby's premature lungs. Now Stephen Lye and Jan Heng at Mount Sinai Hospital in Toronto have developed a test that uses the levels of gene activity - that's messenger RNA or mRNA - to spot women who are about to deliver early, by looking for genes linked to inflammation, which is part of the natural birth process.
But what are the limitations of the current tests for early delivery? Kat Arney spoke to Stephen Lye and Jan Heng of Mount Sinai Hospital in Toronto...
Stephen - Some of them rely on clinical parameters, most notably the uterine contractions, the length of the cervix, how open cervix is, and there are some biochemical tests. Together, those tests are reasonably good at having which women will not deliver, but they're not very good at telling which women will actually deliver within the next 48 hours. And so, they're not really good at selecting the women that you really need to treat.
Kat - Jan, can you tell me a bit about the kind of experiments that you were doing to try and solve some of the problems here?
Jan - So, what we did was we collected blood samples from these women that admitted into the hospital with threatened preterm labour. We extracted the blood samples to get their messenger RNA and with that, we applied the mRNA to microarrays which are tiny chips with DNA probes that reflects the whole human genome. Using that chip, we can screen changes and differential expression of genes in the maternal blood that's associated with impending labour.
Kat - So, you're basically looking for genes that are switched on or switched off when a woman is definitely going to go into labour.
Jan - Exactly. So, that was what we did with the microarrays.
Kat - So, you're looking in the mother's blood for these patterns of gene activity. What sort of things did you find?
Jan - So, we found that these genes were associated with inflammation which is expected because we know that labour is an inflammatory process. It is part of the natural progression of labour where the woman gives birth a term of preterm. What we found was that when they're going to preterm labour, the labour process is also inflammatory response. So, the genes gave us an indication that inflammation was occurring in women who were about to deliver. It also gave us information about high activity that there were more genes being transcribed with impending labour.
Kat - How many women were you actually looking at? Did you find the same pattern of genes in all of the ones that were definitely going to go and give birth?
Jan - We collected the samples from Australia. The study had 154 women. Out of 154, 48 of these women delivered a premature baby within 48 hours.
Kat - Did they all show the same patterns of gene activity? How reliable is this if you're going to develop it into a test?
Jan - From our study, our test can correctly identify 7 out of 10 women that will deliver a baby within 48 hours which is better performing than the current test. The advantage of our test is that it can be performed on most women compared to foetal fibronectin because a lot of women are ineligible for it.
Kat - Stephen, tell me about the next steps for this research. You've tested it on around about 150 women. What next?
Stephen - This study was done in collaboration with Dr. Craig Pennell h in Perth, Western Australia. And so now, what we're doing is collecting similar set of samples here in Toronto, just to make sure that the signature for Perth women is the same as the signature for Toronto. And then we hope to take back to a number of centres around the world including the UK. And then following that, it's really on starting to work on the commercialisation of this to turn it into a diagnostic test.
Kat - How soon would you like to see this being a test that could potentially be really beneficial for mothers and their babies?
Stephen - I would hope within 3 to 5 years. If everything goes well, we will have something that is ready for the market.

12:25 - Coal Mining Dangers
Coal Mining Dangers
This week news broke that Turkey had suffered its worst ever mining disaster after an explosion and fire in a mine in Soma claimed almost three hundred lives.
an explosion and fire in a mine in Soma claimed almost three hundred lives.
But why are explosions such a risk when mining? Here's your Quick Fire Science
- Coal is produced when organic matter, normally plants from swamps or forests, gets buried and then squashed and heated over millions of years
- This causes the organic matter to break down. Gaseous elements like hydrogen and oxygen are driven off, leaving the carbon which forms coal
- These gasses can be trapped in pockets in the coal, but millions of years later, as the mining process splits the rocks apart gases can be released once again.
- Gases can include carbon-dioxide, the poisonous gas hydrogen sulfide, and methane, also known as natural gas, which can explode when mixed with air.
- Methane used to be known as firedamp and was particularly dangerous for miners in the 18th and early 19th centuries when the only source of light was from candles which could ignite the gas.
- Miners used to use canaries and other small birds to detect asphyxiating and poisonous gasses as they would succumb before humans, but methane could reach explosive levels without affecting a canary.
- To solve this problem the British Chemist Sir Humphrey Davey invented a safety lamp after he discovered that a flame wouldn't pass through a fine metal grill. This meant it was possible to burn a candle in a metal gauze tube without igniting any gas outside.
- Davey also found that the flame would change colour and shape depending on gasses in the atmosphere.
- Even the coal itself can be dangerous to miners, because if coal dust is fine enough it will burn very quickly and effectively explode.
- Any explosions in mines can also make the environment more difficult for humans to breathe because the limited oxygen is consumed
- Even more dangerous is the carbon-monoxide produced by burning fuel with limited oxygen, as this binds to the haemoglobin in red blood cells and prevents your blood transporting oxygen.
- In the US over 3200 people were killed coal mining in 1907 to produce about 2 billion tonnes of coal
- However electric light, ventilation, gas sensors, and modern safety equipment has made coal mining far safer than it once was. So in the US in 2012 only 35 people were killed to produce about 600 million tonnes.
- However the safety record in other countries has some catching up to do, in the same year in China over 1300 people were killed mining 2.8 billion tonnes of coal.

15:21 - Gemini Planet Imager: Seeing exoplanets
Gemini Planet Imager: Seeing exoplanets
with Scott Thomas, Cambridge University
This week astronomers announced that they now have a powerful new toy to play with - a system called "the Gemini Planet Imager" that is enabling them to actually "see" planets - known as exoplanets - 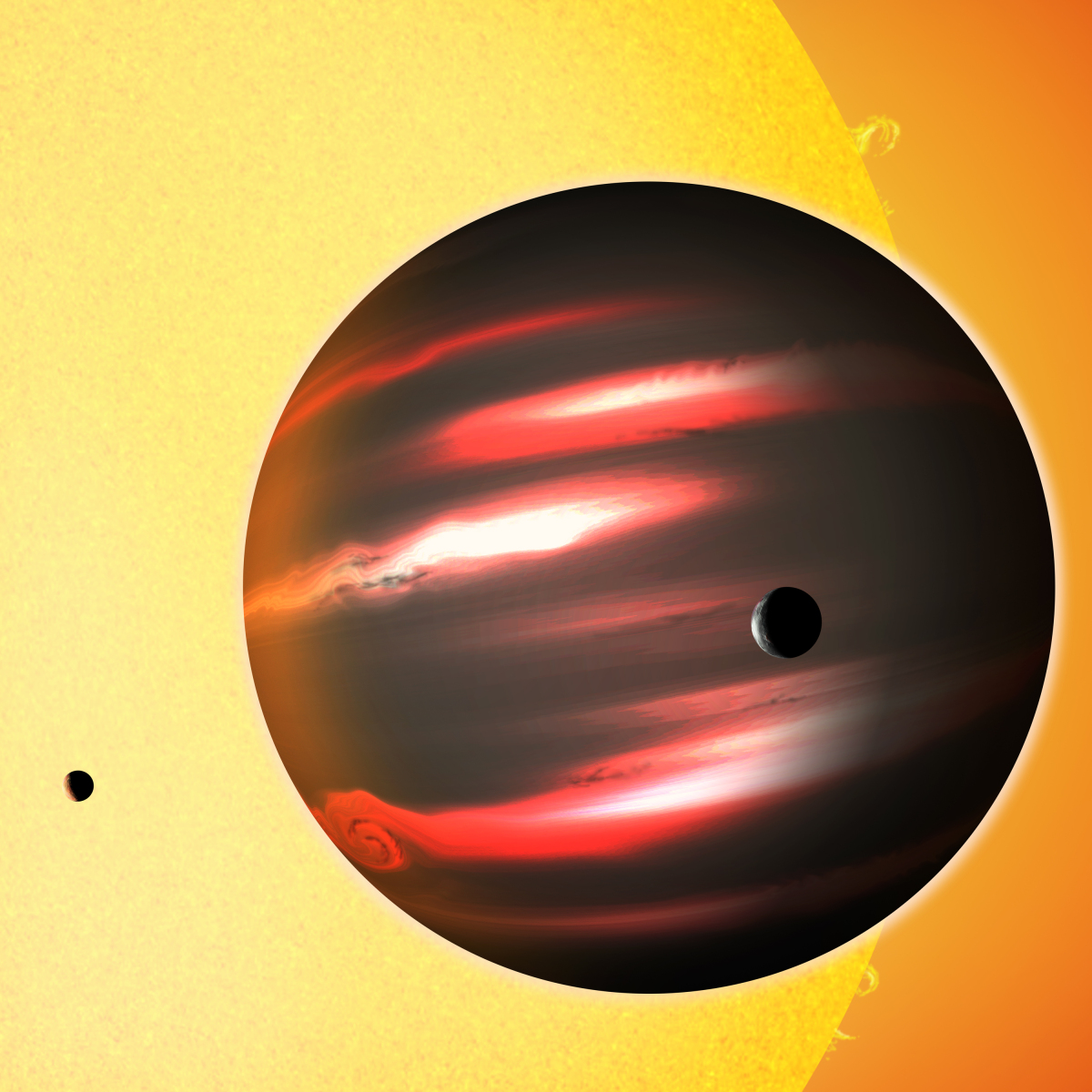
' alt='The distant exoplanet TrES-2b, shown here in an artist's conception, is darker than the blackest coal.' >in orbit around other stars outside our solar system.
Scott Thomas is an astronomer from Cambridge University; he explained the Gemini Project to Chris Smith...
Scott - Extrasolar planets can be spotted in a few different ways. The ones that's been the most successful is called the transit method. You might've heard of the transit of Venus when Venus passed between us and our sun. Well, this is a similar sort of thing. We look at another star and we wait for the planet orbiting that star to pass in front of the star. When that happens, the light from the star dimmed ever so slightly, just a tiny amount - 1%, 2% - we're talking about. And so, that's how we know that something has moved in front of the star and we know there's a planet around it. So, this transit method has been very successful and there's a spacecraft called Kepler which up until it failed quite recently to take possibly thousands of potential planets around other stars. There are other methods as well - the radio velocity method which is where you watch a star and you see if it wobbles very slightly due to the gravitational influence of the planets moving around it.
Chris - And I suppose another constraint of these sorts of studies is that they tell us that a planet is there. We can infer from either the way the light changes or the way that the star wobbles, a bit about what the planet might be in terms of its size and what it's therefore made of. But actually, we can't physically see it, can we?
Scott - Exactly and when people hear about the way that planets are discovered, the first thing we think is, "Oh! We see it on the sky" but that's not just the case. However, the Gemini planet imager are actually able to directly see the planets. So, they're able to look at the sky, look at the star, and produce an image with the star in the middle a little spot next to it, and that little spot is the planet.
Chris - Is the difficulty that the star you're looking at is a billion times brighter than the planet?
Scott - That is one of the difficulties definitely. This instrument uses what's called a coronagraph and a coronagraph is basically when you block out the light from the star, you put not quite a disk of paper, but you've put something over the image of the star to leave only space and the planet around it.
Chris - Sounds pretty easy. Why is this paper published in an international journal this week?
Scott - Well, because there's a lot of engineering that goes into this and in fact, the issue of the star being a billion times brighter than the planet is not the only one. In order to be able to see the planet, the distance from the star we're talking about here is what we call an arc second, an arc second being a very tiny unit of measurement, 1/3,600th of a degree. I think in order to see a planet at the distance from a star is like, trying to see a human hair perhaps at the end of a football field. So, you're dealing with very tiny distances. At these distances, we have trouble with things like atmospheric turbulence. So, any sort of heat, any sort of cloud, anything that's going to distort the air between us on the ground and space where we're looking, that's make it difficult to separate the planet from the star.
Chris - So, with that in mind, where is the Gemini platform and why is that location been chosen?
Scott - The general instrument is on a telescope in Chile because the high deserts there mean that you get a location that's very cloud-free, very clear atmosphere. In fact, it was on the telescope that was already there. I think this is an important distinction to make because when we build a telescope, it's a significant investment in money, in engineering. And so, we want these things to be like Swiss Army knives. We want them to do as much as possible so we'll build a telescope. But we'll make it capable of having several different bits that can be tacked onto the back. It's like if you had a digital camera and you could leave the lens in place, take off the back and replace it with a new camera. So, this new camera in this case is the Gemini Planet Imager.
Chris - What is it delivering? They've turned it on this week. What does it look like it's going to do?
Scott - The press release this week is about an image that have taken of a system called beta pick. Beta pick is a star. The beta means that they sit in brighter star and the pictorous constellation. Beta pick has a disk around it. In this disk, there was a planet. The planet is called beta pick b because we name planets with names like A and B because we're very imaginative as astronomers. And the team behind the Gemini Planet Imager, turned on the Gemini planet and they pointed it at beta pick. Within 60 seconds, they had an image of this planet. So, that's really a pretty astounding sensitivity.
Chris - And what will being able to see these planets at, to what we can currently learn about them just by using existing techniques?
Scott - If you can see a planet directly, you can take a spectrum of it and that means that you can split up the light coming from it and to all its component wavelengths, all its component colours. And then using that, you can draw some information about what's in the atmosphere. So, spectroscopy, the taking of spectrum like this is hugely important for astronomers because it lets us figure out what the components of the atmosphere are. It's the water, it's the oxygen, hydrogen, helium. We see lines in the atmosphere in the spectrum that tell us about this. This is really important because we want to know what these planets are made off, we want to understand something about the chemistry, about how they're formed. Hopefully, tie it back perhaps to our Solar System as well.

21:15 - Why octopuses don't get knotted
Why octopuses don't get knotted
with Guy Levy, Hebrew University in Jerusalem
Why don't octopuses end up tying their arms in knots?
Guy Levy at the Hebrew University in  Jerusalem thinks he has found the answer - a mysterious chemical in their skin. Kat Arney spoke to him to find out why he got involved in this knotty problem.
Jerusalem thinks he has found the answer - a mysterious chemical in their skin. Kat Arney spoke to him to find out why he got involved in this knotty problem.
Guy - It might seem a little weird in the beginning when I'm asking such a question because our arms never interfere with each other and it seems natural for us. But it has to be understood that the unique morphology and structure of the octopus arms being so flexible and with the ability to deform at any point along their lengths, it limits the ability of the octopus to understand and know their exact positions. On one hand, we have all of this, that the octopus is not aware of the exact position of its arms. It cannot see them all the time. On the other hand, it has these hundreds of suckers along each arm that have the reflecting tendency to attach to anything.
Kat - So, you have all these arms going everywhere, the octopus doesn't know exactly where they are. They're covered with suckers that will grab anything. How did you go about investigating how the octopus doesn't tie itself in a knot?
Guy - So, it has to be said that an octopus arm is partially autonomic. That means an amputated octopus arm, a freshly amputated octopus arm will continue to live for more than an hour after amputation. In this time, it will behave. It will grab items. What we did is we tried to put two amputated parts in the same dish. We saw that they don't grab each other. They would grab almost anything or let's say, anything else but not each other. This was the first step to show us that there is a built-in mechanism inside the arms that prevents this unwanted grabbing of each other.
Kat - I have this weird vision of two flailing arms in a dish. What was the next step to try and find out what's actually going on?
Guy - We suspected this has to do something with the skin of the octopus. So, we peeled the skin off one of the amputated arms in the dish. The first one that was not peeled did grab the flesh of the peeled arm. We took the skin and stretched it over a plastic disk and submitted this disk to the amputated arm and it didn't grab it. So then we knew it's something inside the skin that prevents the suckers from using their reflex for attaching.
Kat - So, what do you think it is that's in the skin that repels the other arms of the octopus?
Guy - We suspected it has to be something chemical, but we were not sure but it could have been also something - the texture of the skin. And then what we did in the next experiments is to extract molecules out of the octopus skin in order to see if the extraction, we also prevent the suckers from grabbing. We mixed those molecules inside gel and we coated plastic disks with this gel and we showed that amputated octopus arms indeed avoided grabbing this gel that contained molecules from the octopus skin. But when we did the same thing with fish skin, we embedded molecules that were extracted in exactly the same way from fish skin, then the amputated octopus arm indeed grab the gel.
Kat - So, do you know what this mystery molecules are yet?
Guy - I'm sorry to say that no. We don't know which molecules. This is our next step to try and isolate the precise molecules that are involved in this process.
Kat - This is really fascinating because octopuses are just amazing animals. But why would you want to know how it doesn't tie itself in knots? Is there a wider picture of knowledge that you're trying to build on?
Guy - Other than being very interesting, there are also other implications of this and we are also part of a project of the European Commission. This project is called STIFF-FLOP and it aims at building a soft manipulator that will be in the shape of an octopus for medical purposes. Our part in this project is to provide the biological information of how the real octopus controls its arms and engineers are supposed to take ideas from the biology and implement them inside the controlling system of this soft manipulator.
Kat - So maybe one day, there could be an octopus surgeon inside you that won't tie itself in a knot.
Guy - This project is aiming at building one arm. So, it won't have other arms that it will have to avoid, but maybe this mechanism can be extended and this manipulator can be programmed to avoid manipulating obstacles on the way to the target, in the same way that the octopus is avoiding grabbing its own arms. This manipulator can use similar mechanisms to avoid for example, let's say, it will be used for operations inside the intestines of a human. Then it will have to crawl inside to reach the target. We don't want it to stick to the walls of the intestines on the way.

26:31 - Extreme bacteria lurking in gold mines
Extreme bacteria lurking in gold mines
with Kay Kuloyo, University of the Free State at Bloemfontein
South Africa is home to the world's richest sources of gold and the world's deepest mines that have been dug to access it. But in the course of tunnelling up to 4 kilometres vertically down to recover the gold, miners recently struck scientific gold with the discovery of populations of microorganisms thriving in extreme conditions. The temperatures down there are up to 70 degrees Celsius, and the organisms survive by breaking down minerals and even using the radiation given off by the uranium naturally present in the rock. Chris Smith joined the team collecting samples from seeps kilometres underground...

Kay - My name is Kay Kuloyo. I'm a researcher at the University of the Free State, working to understand the limits of life in extreme environments. We know that we can find extreme life in places like deep sea vents and water sources. And also, with that notion that there could be life on other planets, and where are the places that we feel we can have such conditions, it's in the centre of the Earth. Mining activities have provided that kind of opportunity for us to be able to access these places.
Chris - What's been discovered already?
Kay - In 2010, there was a paper that was published about a worm found in fissure water one of the mines. This is one of the biggest discoveries to date because before that, we only found bacteria. So, that gave an indication that there could be different levels of life in extreme environments.
Chris - When we say extreme environments, where were these organisms found growing?
Kay - We're talking about in the deep mines where there's very little nutrients and the temperatures can go as high as 70 degrees Celsius.
Chris - So, to find complicated life growing at those extreme temperatures and very nutrient-poor environments, it says there's something pretty special about those sorts of organisms.
Kay - Yes. It means that these organisms have genes that can help them make the kind of food they need. In the absence of sunlight and other sources, they can actually use the chemicals and the minerals to make their own food. And so, they have these conditions.
Chris - How did they get here?
Kay - Well, the theory is that over millions of years ago, as the water gradually seeps down, these microorganisms came down with the water and they became trapped here. Over time, they have been able to adapt to the conditions here.
Chris - How do you know that's what happened and that for instance they didn't just arrive with the last rainstorm?
Kay - Okay, well we do isotope analysis to determine the age of the water.
Chris - And what does that tell you?
Kay - That tells us that some of the water samples that we've taken here are millions of years ago and sometimes even up to billions of years old.
Chris - So, if the water is that old, the organisms must've been in it for at least that long.
Kay - Yes.
Chris - You've come down here today. The idea is to actually collect some samples. How are you doing that?
Kay - We have sterile Falcon tubes we always carry which are for grab samples. If we find a seepage in the rocks, we can take some of the water samples and then we  culture in the lab and see what's in there.
culture in the lab and see what's in there.
Chris - Because there's some water dripping down over here. Is this of interest?
Kay - Well, we see water are coming out of the rocks like this. This is probably old water that's seeping out. What you see on the surface of the rocks like the brown colour and the black colour, we think that's a microorganisms that are growing on the rock and also using the nutrients from the water to survive. So over time, they form a film on the rock of different colours - black or brown or white or pink, depending on the kind of nutrients that they're using.
Chris - Do you have a lot of contamination from microorganisms brought here by us?
Kay - Yes, because there's a lot of human activity, mining activity around here. Most times, we find microorganisms that have come from human activities even sometimes common microorganisms like E. coli. So, we have to be really careful what we see is originally from the mine and what's on the surface.
Chris - Shall we grab a sample?
Kay - Yes.
Chris - Get across the railway tracks, not falling in the drainage ditch.
Kay - I will open this sterile falcon tube which is about 50 millilitres and I'm going to scrape some of the water sample from the side and trying to scrape some of the brown stuff. Even very little water samples can yield a lot of information about the microorganisms. With my other tube, I'm going to scrape some of the black colour because we think that could be sulphate which is in bacteria. I don't know if you can smell a little bit of hydrogen sulphide.
Chris - It does smell a bit sulphurous, yeah. Why do you think the sulphur is important?
Kay - Because usually, what you find in the gold mines is a lot of pyrites and sulphates as well and you know, that's also associated with gold. So, a lot of times, we find sulphate reducing bacteria.
Chris - So the bacteria are using the sulphates as a food source.
Kay - (crosstalk)
Chris - Yes, because they're obviously not able to rely on energy coming from the sun. They have to get their food chemically.
Kay - From what's around them, yes. You find some of them as a sulphate, some use iron as well and some use perhaps the nitrates. And also, sometimes they use a radiation.
Chris - Radiation.
Kay - Yes, some of the microorganisms can use the radiation as a source of energy.
Chris - What will you now do with that?
Kay - This, we take back to the lab and we do two types of analysis. One is to see if we can actually culture the microorganisms in there because...
Chris - To grow them.
Kay - To grow them, yes.
Chris - You have to recreate a gold mine in your laboratory.
Kay - We recreate the environment here. We're not always successful because these things do not follow the laws of the laboratory. So, we go another step further to understand what they are made of using molecular biology techniques.
Chris - So, as well as trying to grow them, you can then just interrogate them genetically because sometimes they may not grow, but their DNA will be there nonetheless and that means you can find them.
Kay - Exactly. So, we used their DNA profile to reconstruct what's actually in there to understand all the bacteria, all the sorts of life that are in the samples.
Chris - What are the implications of these discovery?
Kay - Well, because of the conditions that we're finding these  microorganisms in, we are able to use some of these microorganisms for bioremediation purposes.
microorganisms in, we are able to use some of these microorganisms for bioremediation purposes.
Chris - Cleaning things up.
Kay - Cleaning things up like acid mine drainage and things like that. So also, we use them for green technologies, taking away more chemicals and using biological agents. Also for instance, microorganisms that we find in high temperature areas, it means they are able to produce enzymes that are thermostable.
Chris - As the vice president of the company I just pointed out, can you not discover a strain of organism that will eat gold and then pour it out in the right place to make their job a lot easier?
Kay - Well, that's some of the research that we're doing. We have microorganisms that can bioaccumulate the gold and then we can extract the gold from the microorganisms. So, as one of them pointed out that in places where they cannot really reach or it's dangerous, it's possible for us to use microorganisms, that's the next level that we're looking at. We have some bench scale applications of this where we've seen it happen. Not just gold, but copper and some other minerals that can lead to bigger levels and really showing that it can work as what we're looking at now.
(Our thanks to Sibanye Gold's James Wellsted and his colleagues for making this visit possible.)

34:26 - Rare earth metals recovered by bacteria
Rare earth metals recovered by bacteria
with Lynne Macaskie, Birmingham University
Microorganisms might hold the key to the future of precious and rare metal recovery,  and also to detoxify the chemicals that can leach from deposits of mine tailings or "slag heaps".
and also to detoxify the chemicals that can leach from deposits of mine tailings or "slag heaps".
Birmingham University's Prof Lynne Macaskie, works on microbes that can do this. She's recenly been to Cape Town and Canada.
Kat - Tell me, why are these mine tailings or slag heaps such a threat to our environment?
Lynne - They're a big threat because unless the area is contained very well, the toxic elements, the metals and other things can actually migrate away from the source and through the environment and have an environmental impact. So, there's a lot of scope for environmental damage.
Kat - And what kinds of metals? What sorts of contaminants are we talking about and why are they damaging in the environment?
Lynne - Well, the main metal of interest that's being mined obviously whether that's copper, nickel, uranium, but other elements that are there as well which may not be immediately of interest can also have toxic effects on the ecosphere, plant life, animal life. One example of this is where historically, people were mining for uranium and they ignored another set of elements which were often present called rare Earth elements. Now that they in themselves are not too toxic, not as toxic as some are, but they're now becoming quite valuable. So, the interest is refocusing on getting as much benefit as possible from the slag heaps.
Kat - Basically, you've got all these rare Earth metals sitting in slag heaps, why are they so useful? Why do people want to get them out?
Lynne - They're absolutely key to the 21st century technologies. They're used in magnets, electronics, phosphor screens, LEDs, TV screens and a lot of the time, they're not replaceable by anything else. And so, as technology is moving forward, we are coming to rely on these elements. The problem is, not that they're rare. They're actually very common. It's just very, very difficult to get them out and commercial processing is something like a hundred different steps. It's very tricky. It's very expensive. It's been developed in China mainly and so, China largely controls the global supply of these elements.
Kat - So, how are you trying to get these rare Earth minerals out of the slag heaps.
Lynne - As we heard on the piece from Africa, some bacteria can actually take up minerals, take up elements and some will take up gold, some will take up copper. Some will actually take up rare earths. But of course, the tricky part is choosing your bacteria so that effectively, you can put them into different pots. You don't want a complete mixture because then you've got the problem of separating them. So, I think the future lies in getting separation, getting bacteria to take some and not others.
Kat - And so, where are you at the moment with your research? Do you have some bugs in the lab that can actually eat specific metals and minerals?
Lynne - We do have some bugs already. But we're just starting to embark on looking at natural deposits, finding out what bugs are in there. And this story about bugs being found at 70 degrees way below ground is so interesting, so exciting because it's opening up a whole new area of science that hasn't been explored yet.
Kat - Do you think that there are going to be just naturally occurring bacteria that could be useful for this or would you look towards genetically engineering specific capabilities into bacteria?
Lynne - Nature is very clever, given millions of years, things evolve. Quite often, people will genetically engineer a microbe, and then find something else that's engineered itself in the environment. And obviously, there's a lot of resistance still to releasing genetically modified microbes. So by and large, we tend to try for natural microbes where we can.
Kat - What kind of benefits do you think there could be if you could actually successfully get these rare Earth metals out?
Lynne - Well the environmental benefits are obviously strong, but these elements are so, so valuable and the price is going up. Everybody thinks of gold and platinum as precious metals. But actually, we're not short of gold and platinum. There's plenty of that, but we don't yet have the technology to harvest rare earths from the environment in an efficient way, that's cost-effective. So potentially, there's a lot of economic benefit to be gained. And in fact, in parts of Canada, they've got huge areas of tailings ponds where they've taken out the uranium for the nuclear industry and they're left with huge tailing ponds full of these rare earths. At the moment, they're seeking exploitation licenses to go back to these historic deposits.
Kat - I was going to say, it sounds like they're sitting on a gold mine, but obviously, it's a rare Earth mine. In terms of just getting the bugs in and out, would you just be taking bacteria out and purifying them or isolating bacteria, bunging them in there, making them grow, and then taking them back out again.
Lynne - Well, it's a very large problem obviously. It's not just a small hole. It's a very, very big hole. So, there's a lot of technology to be developed to try to make it a manageable size. And part of the bottle neck is, it's quite difficult to do pilot plants because you've got to construct a smaller version to try out. Also, the actual composition of the material differs from mine to mine.
Kat - What kind of timescale briefly are we talking about here that you could maybe start getting metals out? What would you hope for?
Lynne - We would hope to start getting the metals out and proving the technology in principle within 2 to 3 years. But obviously, getting it up to plant scale will be much longer than that, somewhere between 5 and 10 probably. And don't forget also that you have to convince the wider public and the governments that you're not going to end up making a larger mess you started with.

40:37 - Managing oil spills with bacteria
Managing oil spills with bacteria
with Joel Kostka, Georgia Institute of Technology
This week we've been talking about bioremediation, how bacteria can be used to clean up environmental damage in soils and mining waste. But can the same techniques also be used in our oceans?
Joel Kostka is from the Georgia Institute of Technology  and investigated in how microorganisms played a role in cleaning up the aftermath of the Deepwater Horizon Oil Spill in 2010.
and investigated in how microorganisms played a role in cleaning up the aftermath of the Deepwater Horizon Oil Spill in 2010.
Joel - In the past, the accidental release of oil into the environment largely occurred by tankers running aground and breaking up into shallow water. But in recent times, oil tankers have become much better designed. And so, even if a tanker runs aground, there's less of a chance for that ship to break apart and release oil into the environment. So more and more, what we're seeing is oil is released from pipes, associated with these floating oil rigs like the Deepwater Horizon rig in the Gulf of Mexico that drill through miles of seawater and miles of ocean floor. What we say in our field now is that it's not a matter of whether we will have another major oil spill. It's a matter of when.
Chris - When the disaster happened with Deepwater Horizon, how much oil was actually being lost or discharged into the Gulf of Mexico?
Joel - The total oil released was about 5 million barrels of liquid oil and one third as much again of natural gas is released into the deep ocean. So, we're talking about over 6 million barrels of oil equivalents were released into the ocean.
Chris - Now, you work on microbes and how they are affected and how they affect oil and water. So, when you go and look at a sample of water from the Gulf of Mexico, are the ocean waters richer in bacteria there that will naturally be able to degrade oils?
Joel - Certainly. So, one of the unique aspects of the Gulf of Mexico is though it's vast, it still is relatively small ocean basin, into which a lot of oil is deposited from natural oil seeps. And so, if you take samples with the submarine or another device around where that oil is seeping out, you will indeed see a concentration of microbes that have evolved to take up and eat that oil. Bacteria that have evolved to eat oil carry out what's called a respiration process. They breath oxygen and burn the carbon that present in the oil. So, oil is an excellent food source for microorganisms.
Chris - And so, the logical question for me to ask is, given that those bugs are there, can you recover them, enrich them and then use them if there is another oil disaster to help degrade the sleeks in some way?
Joel - I wish that was the case, that it would be that simple. There's really credible evidence that indicates any organism that we can grow in the lab and add into the environment that would do a better job of degrading oil hydrocarbons than the indigenous or native communities. The reason for that is that the microbial communities that are present naturally in seawater or at the seafloor are adapted better to those environmental conditions that are there than anything that we can add.
Chris - What are these microorganisms? What sorts of bacteria are they?
Joel - Some of the best known organisms that we've studied so far are members of what we call the gammaproteobacteria and just from their names, you can tell that they're good at breaking up oil for example. Probably the best studied organism worldwide is called alkanivorax. It's named alkanivorax because it eats alkanes. Another organism would be called (marinobacteriahydrocarboneclasticus) and again, an organism that breaks apart hydrocarbons.
Chris - Now, in a human context, if I had an unsettled stomach or I'd had a course of antibiotics which had wiped out the bugs that naturally live inside me and help me breakdown my dinner, I could supplement myself with some bugs or eat certain foods which would encourage certain microorganisms to increase in number. Can we do the same thing in the ocean? Knowing that we've got in our pollution problem, could we help to bioremediate by fertilising the right sorts of bugs in order to encourage them to breakdown the nasty stuff faster?
Joel - That's a good question. So, in the response to the Exxon Valedez spill, the largest response that was undertaken was to fertilise beaches that were impacted by oil. Certainly, it was observed that the oil was degraded faster than it would've been if the beach had not been fertilised. But it remains controversial as to whether that remediation strategy was really effective because the oil probably would've broken down naturally without fertilisation. And whenever you add nutrients to the environment, you can potentially cause some unintended consequences like for example harmful algal blooms.
Chris - So, what would you say the bottom line is with respect to the use of microbes in cleaning up oil spills?
Joel - I would say that the use of microbes in cleaning up oil spills is that natural microbial communities provide ecosystem services really all over the globe, it's a supply and demand issue. As long as the release of oil into the Gulf of Mexico is slow enough, natural microbial communities can keep up and degrade any oil that's released into the environment. It's only when you have a huge amount of oil released in a short period of time. So, much higher supply that it outpaces the demand and the microbes can't keep up. But eventually, that oil will be degraded naturally. It actually occurs fairly rapidly in the presence of oxygen and in the presence of sufficient nutrients.

46:26 - Fungi, nature's recyclers
Fungi, nature's recyclers
with Geoff Gadd, University of Dundee
So we've heard how bacteria can play a role in environmental clean ups, but what  about nature's recyclers, fungi?
about nature's recyclers, fungi?
Geoff Gadd from the University of Dundee works on this question.
Geoff - We've been working on various systems and we've been particularly interested in lead and uranium in some separate studies. In fact, we've found that in both cases, fungi are capable of mediating the formation of lead-containing minerals or uranium containing minerals. They're actually some kinds of phosphates. In fact, it was very interesting with the lead because the mineral formed was a lead phosphate called pyromorphite. Now, this is a mineral and it's actually the most stable lead mineral that's found in the Earth's crust and was commonly advocated as a remediation technique for lead contaminated land. Phosphate sources such as apatite or bone meal would be added and this would immobilise the phosphate. But we've shown that the actual organisms themselves can actually form the pyromorphite in the soil and it could be just a case of encouraging the fungal populations to grow up a bit to do this.
Kat - So basically, lock it away in a form that it can't leech out and contaminate the wider environment.
Geoff - That's the idea and it's actually the same with uranium. They made a uranium phosphate mineral and funnily enough, the uranium phosphate mineral was actually more stable than the depleted uranium that was being attacked by the organism. So, this might be one good approach where the metals are locked up in an insoluble form and then hopefully, they don't leeched out and find their way into water or get taken up by plants and the like.
Kat - Where are you looking for these wonderful metal converting fungi in the world?
Geoff - Well, funnily enough, you can really get organisms everywhere that can do these kind of tricks, but I will say that we did find some special fungi over in old lead polluted mine sites in the southwest of Scotland, actually near a place called lead hills. We found that some organisms isolated from there were the ones that had this capacity to transform lead into this mineral. So, in some cases, there might be a connection between some kind of special organisms and a kind of polluted location where they've adapted or they've been selected because of this property. They can also help them by removing the lead toxicity.
Kat - And thinking about how you might try and take these fungi that you've found say, in Scotland and take them to other sites that are contaminated, would your idea be that you kind of grow them up in the lab and then ship them out? Or are there other ways that you could maybe encourage the naturally occurring fungi there already?
Geoff - Addition of organisms is one possibility although that's often done for some kind of organic breakdown. But in actual fact, it's best to use the indigenous populations perhaps by encouraging them. Some of these things are widely found properties in many species, in fact, just the additional of fertiliser basically just the same way as you might put manure on your garden to encourage plants to grow - the same approach, providing carbon and nitrogen to enable fungal populations to grow might work. In fact, this has been shown over in the US some time ago with cases of selenium pollution where the organisms were encouraged to grow by addition of such materials and remove of selenium pollution.
Kat - So you're basically just giving mother nature a little kind of a nudge in the right direction.
Geoff - Exactly.
Kat - So, do you feel that the future is fungal or do you think we're just going to have to use as many different cleanup methods as possible to try and decontaminate our environment?
Geoff - I think there's possibilities in all contexts. I mean, bacteria have a fantastic metabolic capability and so, they're applicable to a whole kind of situations. Fungi are perhaps are a bit more limited in the kinds of environmental places they can be, but there's still a wide range of possibilities. And of course, we mustn't forget that in a polluted site, especially polluted soil, both bacteria and fungi are together. In fact, it's been shown that both bacteria and fungi have to work together to treat some of the more complex aromatic organic pollutants for example. So, everything is possible.
- Previous Addressing Autism
- Next Which painkiller should I use?











Comments
Add a comment