Scientists have developed a gene editing system with the potential to cure the inherited blood disorder sickle cell anaemia.
About 250,000 children are born with sickle cell dis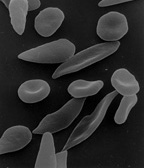 ease every year. It's an inherited condition affecting one of the genes coding for the oxygen carrier molecule haemoglobin which is present in our red blood cells.
ease every year. It's an inherited condition affecting one of the genes coding for the oxygen carrier molecule haemoglobin which is present in our red blood cells.
The condition is particularly common in black Africans because carriers of the disease, who have one healthy haemoglobin gene and one sickle-afflicted haemoglobin gene, are largely healthy but are also protected against malaria. So, over evolutionary time, this advantage has boosted the prevalence in the population of the mutant gene.
But individuals with mutations in both of their haemoglobin genes develop "sickle cell disease" and suffer a range of health effects, including bone and joint pains, damage to their internal organs and low blood counts that lead to fatigue and poor exercise tolerance.
This is because the mutant haemoglobin has a tendency to clump into rod-shaped aggregates, deforming the red blood cell into a sickle shape. This shortens the lifetime of the red blood cells, leading to chronic anaemia.
The affected cells can also become lodged inside small blood vessels, causing blockages to the blood flow that painfully starve and injure the tissues downstream.
Sickle cell sufferers often depend upon blood transfusions and drug therapies, but these have significant side effects and a person's life expectancy is often impacted.
Now researchers at the University of California, Berkeley, have developed a new treatment that could offer these victims a cure.
Writing in Science Translational Medicine, Mark DeWitt and his colleagues have managed to correct the sickle genetic mutation in blood-forming stem cells collected from sickle disease patients.
First, working with cells in a culture dish, they used the CRISPR-Cas9 gene editing system to scrub out and "over-write" the genetic error in the cells' haemoglobin genes.
The corrected cells were then grown in culture where they matured into blood-producing cells called erythoblasts, which were tested for haemoglobin production.
Over half of the haemoglobin coming from these cultures was now a healthy adult form of the molecule. Unedited control cells, on the other hand, continued to produce predominantly the mutant sickling haemoglobin form.
But the question of whether cells edited in this way might survive in a patient remained outstanding. So the team injected some of their edited stem cells into mice in which the immune system had been switched off to prevent the human cells being rejected.
Four months later these stem cells, or their progeny, were still detectable in the bone marrows of the animals, proving that the editing process can produce long-term viable cells.
So the approach envisaged by the Berkeley team is one of extraction and treatment outside the body of a sample of a sickle patient's bone marrow stem cells, followed by re-infusion of the edited cells. These will find their way back to the bone marrow and begin to produce healthy blood cells.
"There's no problem with immune incompatibility, because these are the patient's own cells," says DeWitt. "And now we know this works, we need to work towards a clinical trial in human patients..."










Comments
Add a comment