The Barking Dog Experiment
Ingredients
A tube is filled with nitrous oxide gas (N2O) and this is mixed with carbon disulphide (CS2). The tube is then put in a safety screen and ignited, with impressive results.
| |
| Barking Dog Reaction Video |
Explanation
The reaction between nitrous oxide gas (N2O) and carbon disulphide (CS2) releases a large amount of energy, as the CS2 reacts with the oxygen in the nitrous oxide to form carbon dioxide (CO2), sulphur dioxide (SO2)and some pure sulphur which is left on the tube at the end.
Most of this energy is converted into heat, but far more than normal is converted into light. This is why it is so blindingly bright and is due to a process called chemiluminescence.
Chemiluminescence is what produces the light in glow sticks, it basically means that energy goes directly from chemical energy to light energy without heating anything up. In this case it is probably due to the reaction of two intermediate products of the reaction, sulphur monoxide (SO) with N2O to form SO2 and N2
The SO2 is created in an excited state with lots of energy which it releases as light.
Why does the reaction make so much noise?
If you look at these very slowed down (by a factor of 200) videos of the reaction you will see that the reaction oscillates as it moves down the tube, this oscillation will vibrate air in the room causing the loud noise.
| | | |
|---|---|---|
| A 'small' 1m barking dog reaction | A larger 1.5m reaction. Note the rather beautiful 'smoke rings' it produces from the top | A large 2m tube which had strength issues at the end. |
The reaction sometimes gets extremely violent at the end. This is partly because the pressure variations at the bottom of the tube are going to be large because the momentum of all the gas in the tube vibrating is compressing the gas at the bottom. This speeds up the reaction a lot, which will increase the pressure, which will speed up the reaction, depending on the size of the tube this effect can run away producing a very violent reaction at the bottom, as you have seen sometimes the tube is not strong enough to take this build-up in pressure.
Something similar happened to Liebig who discovered this reaction. Either his tube wasn't strong enough or some NO2 crept into the tube which make a much more violent explosion, and the queen of Austria was quite badly cut by a piece of flying glass. Dr Hal had set up much better safety screens than Leibig did so we were all fine.
Why does the reaction oscillate?
The rate of this exothermic reaction is very dependent on pressure, probably the rate of reaction is proportional to the pressure cubed, so if you double the pressure you will increase the rate of reaction by a factor of 8.
This means that if there is a small increase of pressure in the tube the rate of reaction increases producing more heat and more hot expanding gasses. These will increase the pressure even more.
If you did the reaction in an empty field the gasses could escape easily and all you would get is a small whoosh or bang, but because the experiment was done in a tube, the increase in pressure will start to push gasses out of the tube. These gasses have momentum so once they have started moving they will keep going, overshooting and actually reducing the pressure in the tube.
This would cause the air to start rushing in again and the gasses will start rushing back in again and the tube would resonate. But because the drop in pressure will slow the reaction, the drop in pressure is magnified. This means that increases and decreases in pressure due to the vibration are magnified by the changing speed of the reaction, so the vibration gets stronger and stronger.
 |  |  | 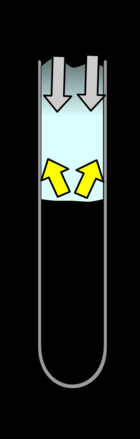 |
|---|---|---|---|
| As the nitrous oxide reacts with the carbon disulphide they release lots of energy: some as light but most as heat. This increases the temperature of the gasses and causes them to expand | The expansion causes gas to start moving out of the tube | The gas having momentum will tend to overshoot as it runs out. This reduces the pressure where the reaction is occurring and slows the reaction down, further reducing the pressure. | The gasses rush back in towards the area of low pressure. This increases the pressure and increases the rate energy is released. Thus starting the cycle again slightly more strongly. |
Why are the oscillations not at a constant speed?
I think the reaction is putting energy into different modes of vibration in the tube (harmonics) and which mode is being excited changes depending on how far down the tube the reaction has got. But I need to do more calculations to be sure...
Thanks to Dr Hal (
www.drhal.co.uk) for doing the experiment for us.
- Previous Weighing Buoyancy
- Next Phosphorus Moon










Comments
I thought it was suppose to
I thought it was suppose to bark. what is up with that!?
The oscillations of the flame
The oscillations of the flame front cause soundwaves, and the soundwaves are what sounds like a barking dog
Add a comment