Ingredients
 | Some batteries of different types (zinc carbon work the best) |  | A plastic bag or container |
| A freezer |  | A torch - (ideally one where it is possible to open the battery compartment whilst it is running) | |
 | a multimeter (if you have one) |
Instructions
Take two identical batteries. Put one in the freezer for a couple of hours, and keep the other at room temperature.
When you take the one out of the freezer, see if it works the same as the warm battery. Does it have the same voltage? Does it work in a torch as well?
If you have any other types of batteries, eg rechargeable, zinc carbon, alkaline etc. see if they work the same.
Result
You should find that the voltage of the battery hardly changes to start with, but if you put a cold alkaline or zinc carbon battery in a torch, it doesn't work nearly as well, and the voltage drops.
 |  |
| A warm zinc carbon battery produces about 1.7V | If you draw a current with a bulb the voltage drops slightly to about 1.5V |
 | 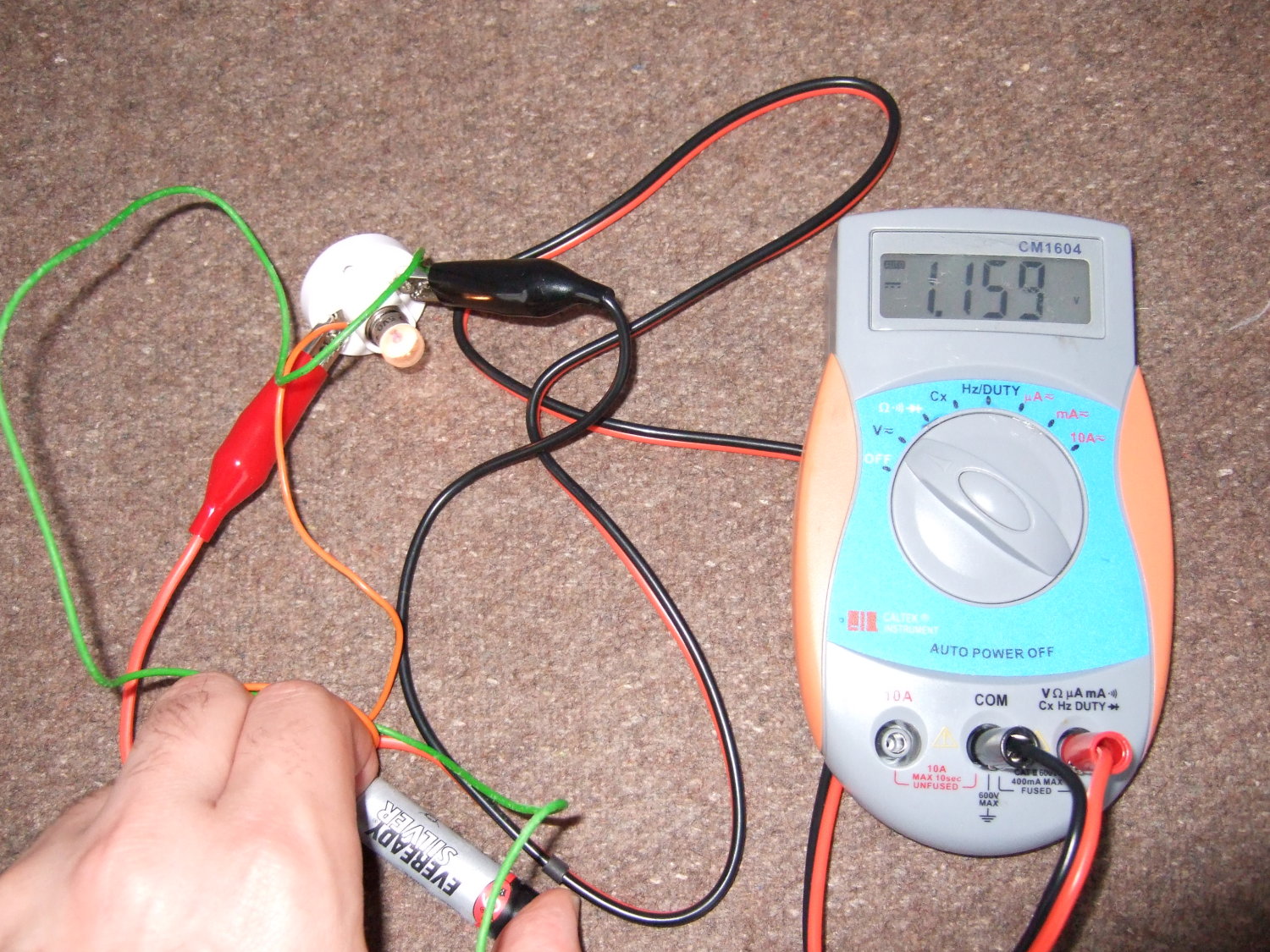 |
| The cold battery produces virtually the same voltage as the warm one. | But if you draw a current, the voltage drops quickly. |
Explanation
A battery is a device for converting chemical energy into electrical energy, It uses a reaction which adds electrons to the negative electrode, and removes them from the positive one. The reaction will continue to do this until there are so many extra electrons on the negative electrode repelling the new ones that the energy required to add an electron is more than the energy released by the reaction.
This repulsion is also a measure of how hard electrons will be pushed around a circuit, and is called the voltage. This means that the maximum voltage produced by a battery is set by the type of chemical reaction that is going on, and so it isn't affected by much, until the battery is almost flat.
This isn't, however, the voltage you get out of a battery if you actually use it. As soon as the electrons are allowed to flow around the circuit to the positive end, the voltage it can produce is slightly reduced because the reaction has a maximum speed, and it can't replace the electrons quite as fast as you can use them.
The more current you use, the bigger this voltage drop is, and this is why you couldn't start a car with 12V of AA batteries - they couldn't produce enough current.
If you cool the battery down, you will slow down the chemical reaction (see the
freezing glow sticks experiment) so reduce the amount of current it can provide, and therefore reduce the voltage it will produce when attached to a light bulb.
All batteries will slow down as they get colder, but some such as Nickel Metal Hydride batteries can provide very large currents to start with, so you might not notice the effect unless you plug them into a very large torch.
This is why you will find that your batteries on your digital camera don't last as long on a very cold day, and why on space missions they have to have special heaters to keep the batteries working.
How does the chemistry work?
Batteries use chemical reactions which involve moving electrons from one type of atom or molecule to another. These are called redox reactions. An example of this form of reaction is rusting; iron reacts with oxygen to produce positive iron ions, and negative oxygen ions.
2 Fe + 3O2 → 2Fe3+ + 3O2-
This sort of reaction occurs because more energy is released by the oxygen gaining electrions than is used up by the iron loosing them.
Battery designers take advantage of this sort of reaction by splitting it in two. For example, in an alkaline battery, the zinc looses electrons on one side and water gains them on the other, but because they are separated, the reaction can only occur if electrons can move from one side to the other through your circuit.
On the positive side:
2MnO2 + H2O + 2e-→ Mn2O3 + 2OH-
On the negative side
Zn + 2OH-+ → ZnO + H2O + 2e-
The reaction can only proceed if charge can move around your circuit, and as energy is released, the charge is pushed around.
- Previous Fruit Fireballs
- Next Why does a pigeon's head bob as it walks?










Comments
What concept could be used
What concept could be used here
Not sure what you mean?
Not sure what you mean?
Add a comment