Ingredients
A large glass or jug
Bicarbonate of soda
Vinegar
A candle
Instructions
Put 3-4 teaspoons of bicarbonate of soda in the glass
Add a cm or so of vinegar
Wait for the foam to die away.
Light the candle
Imagine the glass was full of a liquid, and gently poar the imaginary liquid onto the candle flame.
Result
You should find that the candle gets put out without you having to touch it.
Explanation
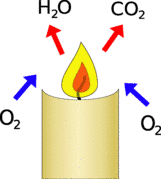
To burn the candle takes in oxgen (O2) reacts it with the candle wax to produce carbon dioxide (CO2) and water (H2O).
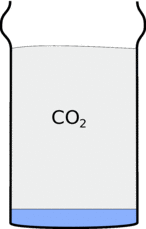
Locked up in the Bicarbonate of soda is a gas called carbon dioxide (CO2) and When you add the vinegar this is released, filling up the glass with carbon dioxide. This carbon dioxide is heavier than air so it stays in the glass, and if you tip up the glass it will pour out and fall down in a stream.
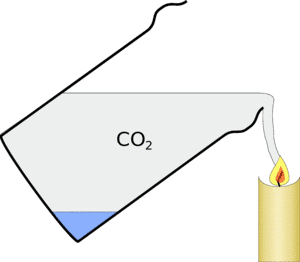
As carbon dioxide tends to exclude the oxygen from the candle and is a waste product of its burning the candle flame can't burn any more and goes out.
Carbon dioxide fire extinguishes work on this principle - they contain compressed carbon dioxide and when you use one on a fire, they exclude the oxygen and make the burning less efficient and put it out.
- Previous Butter from cream
- Next Boiling Yoghurt Pots










Comments
Add a comment