Lemonade Volcano
Ingredients
A bottle of fizzy drink (soda), ideally refrigerated.
4-5 white powdery mints - eg Polos, Mentos, or extra strong mints
A paper tube or if using polos a rod that will fit in the middle eg. the centre of a biro.
Instructions
You want to get all the mints into the bottle as fast as possible so you need a way of dropping them all together. If your mints have a hole, you can thread them all on a rod, otherwise roll a paper tube slightly larger than the mints, but smaller than the hole in the top of the bottle.
Very carefully open the bottle, trying not to let it fizz.
Hold the rod/tube of mints over the bottle.
Release the mints quickly, and retire to a safe distance!
Result

The drink should turn to foam and errupt out of the bottle in a satisfying column up to a metre high.
If you constrict the hole in the top of the bottle by drilling a hole in the lid you can get it to spurt 2-3m into the air.
Explanation
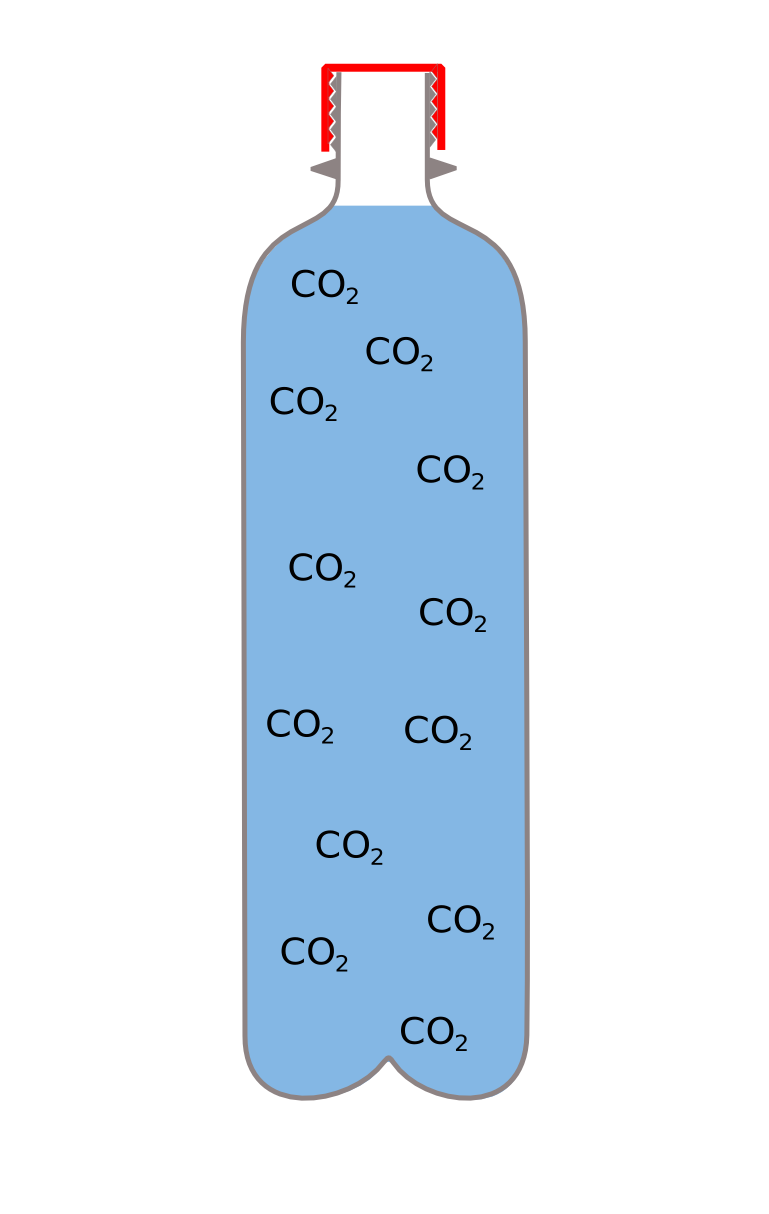
A drink is fizzy, because it has lots of carbon dioxide ( CO2 ) dissolved into it under pressure, while the pressure is maintained the CO2 stays in solution. When you open the bottle the pressure is released, and the CO2 starts to come out of solution.
 | 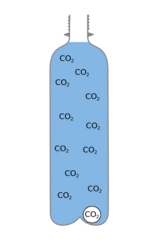 | 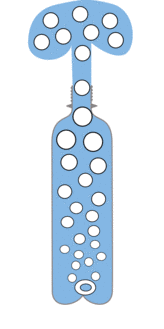 |
| There is lots of CO2 dissolved in the drink. | When the pressure is released the CO2 wants to escape but can only do so slowly. | If you add mints bubbles can start easily turning the whole drink into a much larger volume of foam |
The bubbles need something to start to form on though and because the inside of the drinks bottle is very clean there are very few places where they can form, so it will take a couple of hours for the CO2 to be released.
The mints are really rough, so when you add them, there are lots of places for the bubbles to form (if you added salt, sugar, sand etc it would work too) so loads of bubbles are created all at once. This makes the liquid hugely bigger forcing it to escape rapidly out out the top, in an erruption.
Why do the bubbles need something to start on?
Surface tension is the force that pulls water together into drops, and also is trying to compress bubbles.Whatever the size of the bubble the suface tension is the same.
A small bubble has a very curved surface which means that much of this force is acting inwards compressing the gas inside.
A larger bubble is much less sharply curved so less of the force is acting inwards so the pressure is lower.
![]()
If a bubble gets small enough the CO2 inside is at such a high pressure that it will dissolve again, causing the bubble to collapse.
This means that there is a critical size of bubble, bubbles bigger than this grow and bubbles smaller shrink. So normally no bubbles can form, however if you drop a mint in water the rough surface will make it easier for bubbles to form, as water doesn't stick to sugar as well as other water, and because it will drag little air bubbles along with it trapped in amongst the rough surface.
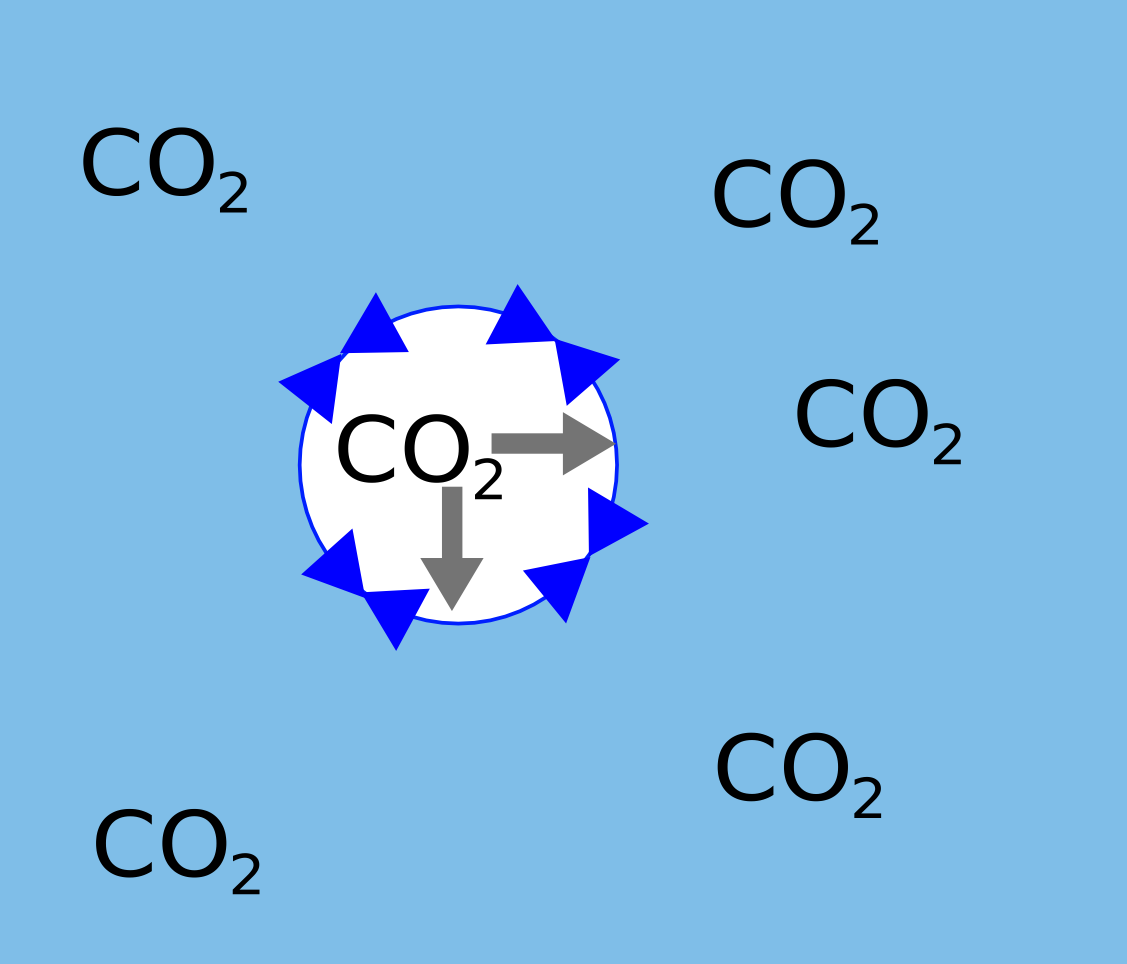 | 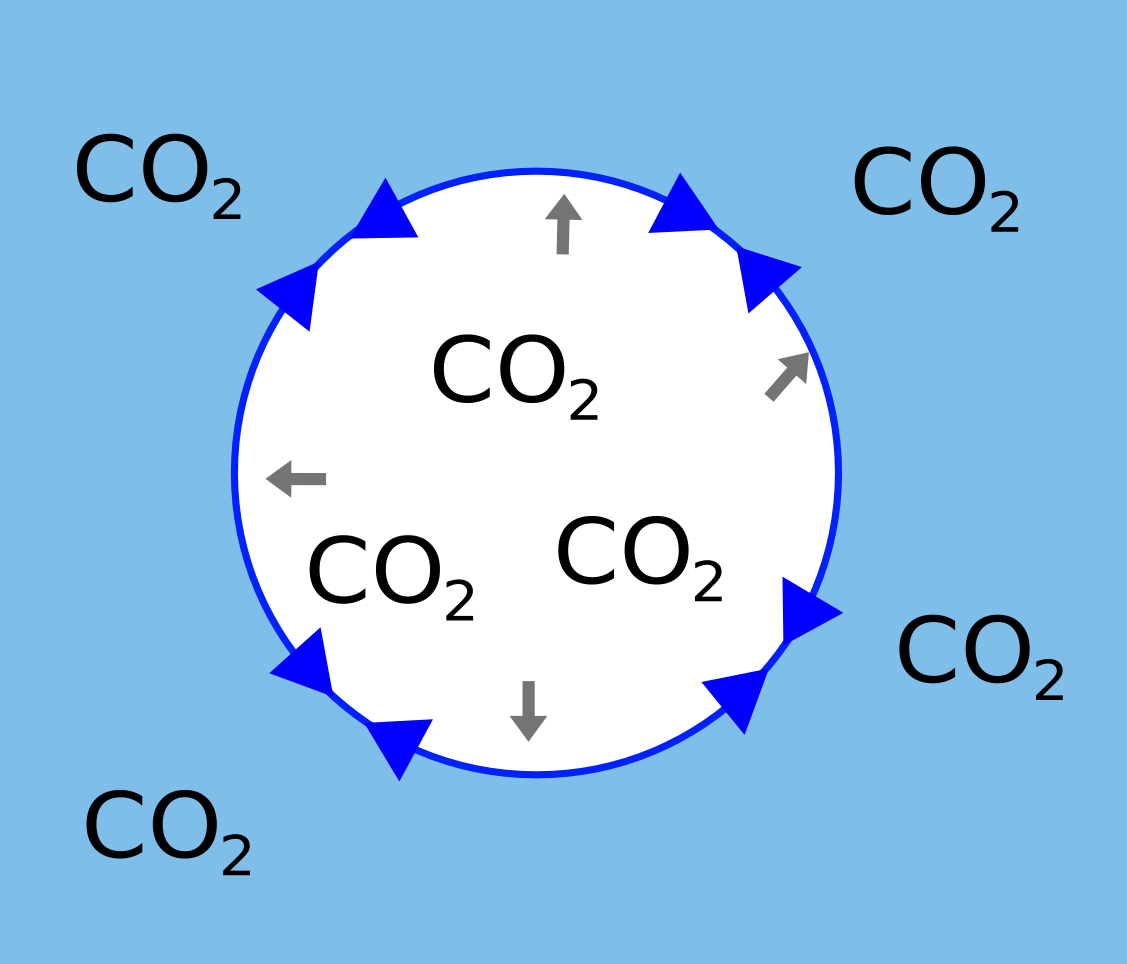 |
| The pressure is so large in a small bubble the CO2 will dissolve again. | In a large bubble the pressure is lower so the CO2 can move into it causing it to grow. |
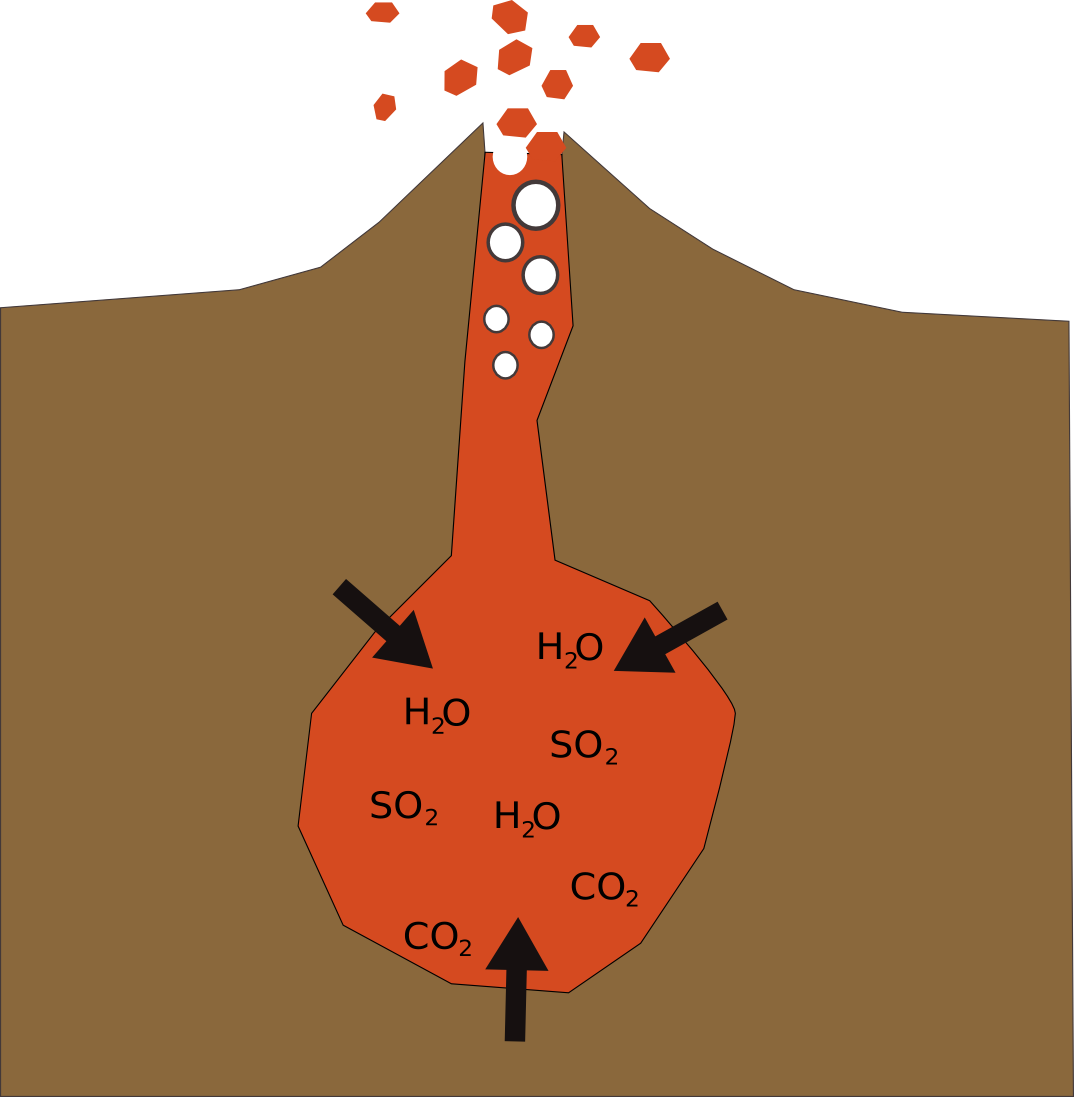
What has this got to do with volcanoes?
There are many types of volcano, but the ones that errupt explosively, have lots of gas dissolved in the magma. If the pressure builds up and then is suddenly released, these gasses form bubbles, and expand to thousands of times their original volume throwing, rocks, ash and pummice kilometers.
- Previous Racing Jam Jars
- Next Fire Piston










Comments
Add a comment