Ingredients
A hard boiled Egg
A bottle with a neck slightly narrower than the egg
Some boiling hot water or a lit match
A tray
Some oil to lubricate the outer surface of the egg
Instructions
Peel the egg and lightly oil the outside to lubricate it
Then either:
Light a match and put it in the jar
...or...
Put the jar in a tray (the jar may crack in the next stage so this will catch any spillage of hot water)
Add a little boiling water to the jar
...Then quickly put the egg on the top and wait.
Result
You should find that the egg is slowly sucked into the bottle.
Explanation
When you add the match or the hot water you cause the air inside to expand, this will push the egg up slightly and some air will escape.
As the air then cools down again it starts to shrink, this pulls the egg down and forms a good seal. This means that the air pressure inside the bottle will reduce allowing the air pressure outside to push the egg into the bottle. And because a boiled egg is very rubbery and flexible you can get it into a bottle that has a neck far smaller than you would expect.
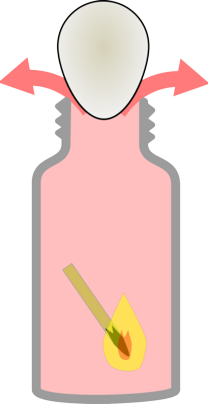 | 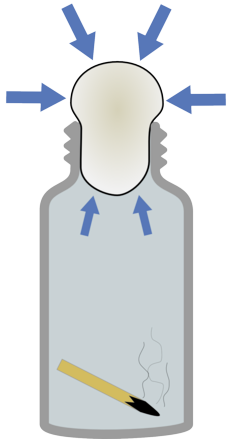 |
When you add the match the air heats up and expands | When the match goes out the air shrinks reducing the pressure inside the bottle. |
How do I get the egg out again?
Though there is always the simple mash it up and take it out in pieces option, there is a much more elegant and scientific strategy. Hold the bottle upside down so the egg falls over the neck, then blow into the bottle as hard as you can, the egg should then be pushed out by the higher pressure air inside the bottle, so you can still eat it for your tea.
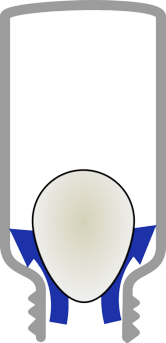 |  |
| Blow air into the bottle around the egg. | This raises the pressure inside the bottle which pushes the egg out again. |
- Previous Lighting bulbs without wires
- Next Fizzy Yeast










Comments
can you make the egg hard
can you make the egg hard again?
No! Unfortunately, this is an
No! Unfortunately, this is an "eggs-ample" of a one-way journey!
nice
nice
What helps the egg to pull
What helps the egg to pull down into the bottle??
Is the oil or the air pressure
How does this experiment link
How does this experiment link to gas behaviour and gas laws?
Thank you
Add a comment