eLife Episode 11: Pain, gene therapy, and regenerating worms
In this episode of the eLife podcast we hear about neuropathic pain, gene therapy, insulin production, ageing in worms, and how flatworms grow new body parts.
In this episode
00:40 - Getting to grips with neuropathic pain
Getting to grips with neuropathic pain
with Steve Prescott, University of Toronto; Stephanie Ratte, University of Toronto
Neuropathic pain is a debilitating nerve condition in which patients can experience excruciating pain in the absence of any obvious tissue injury. Clearly, the pain pathways concerned must be more active than they should be, but no one actually knows why. Now, a new paper by Stephanie Ratte and Steven Prescott who are both at the University of Toronto, has shown by using drugs to tweak the activity of cultured neurons that it's not simply one single condition. There are lots of ways that nerve cells can become over-excitable.  They spoke to Chris Smith...
They spoke to Chris Smith...
Steven - Neuropathic pain is pain caused by damage to a dysfunction of the nervous system. It can result from nerve injury or certain types of diseases - shingles or diabetic neuropathy. Problem is that pain signals arise abnormally from within the nervous system or that they're amplified within the nervous system rather than being accurately relayed from the skin or muscle where they normally originate. So, we were really trying to understand how that originates from changes in the nervous system in the nerve cells themselves.
Chris - So Stephanie, how have you gone about trying to make this attractable problem?
Stephanie - Excitability of nerve cells that we know changes in neuropathic pain is regulated by ion channels. These ion channels are proteins in the cell membrane that control the movement of ions in and out of the cells. So, ion channels are directly responsible for controlling degeneration of spikes and neurons. And spikes are the electrical signals that neurons use to transmit information in the nervous system. After injury, many neurons start producing more spikes than normal. We know from previous work that there's many ion channels that are upregulated or downregulated after injury. But the connection with the hyperexcitability is more of a correlation. So researchers have looked at neurons after injury and they've observed that the density of different channels in the membrane is changed. But that doesn't mean that those changes in the ion channels are actually what causes the hyperexcitability of the neuron.
Chris - So, can you disentangle that?
Stephanie - So, we decided to take a different approach than previous studies. And so, rather than triggering the changes in ion channels by injuring the neurons, we took healthy neurons and we try to reproduce those changes in ion channels in those healthy neurons. So this way, we could see what the impact of the individual ion channel changes would be under cellular excitability.
Chris - And you did that by adding various drugs in things in order to manipulate the ion channels artificially to produce that spiking activity.
Stephanie - That's right.
Chris - Do you know if that directly translated into a phenomenon that would be equivalent to neuropathic pain syndrome in a human?
Stephanie - Well, we don't know because we're looking at individual neurons. We're not looking at the entire system and we don't have a response. We don't know if it's actually causing pain or not, but what we do know is that the changes that we apply to the neuron causes or does not cause the hyperexcitability or the increase in spikes. So, that is all that we can say, but these increasing spikes has been observed in neuropathic pain.
Chris - So, when you pharmacologically manipulate these cultured nerve cells, you can end up with spiking activity that is suspiciously similar to what we see in the in vivo situation when an individual has a neuropathic pain state.
Stephanie - That's correct.
Chris - Your deduction would therefore be that the ion channel changes come first and then the pain state comes secondary to that.
Stephanie - That would be the assumption, yes.
Chris - So Steve, given what Stephanie has been saying in these observations, this therefore suggests that when we see a neuropathic pain state, actually, there could be multiple different underlying mechanisms all happening at once, triggering the observed phenomenon in any given individual patient.
Steven - Right. One way of looking at this is that there seems to some sort of tipping point in the neurons. So, we can artificially upregulate one sort of channel or artificially downregulate another sort of channel, and not necessarily have any effect on excitability until we reach some sort of threshold at which point the excitability of this neurons sort of dramatically changes. And this neuron now fires really like gang busters after we've reached that threshold. And the second finding is that there's actually many different ways of reaching that tipping point. And that has some important implications. What it tells us is that there are in fact many different individual changes sufficient, in and of themselves. There's no single change that is uniquely necessary. Ideally, from a therapeutic perspective, what we really like to do is to hit that one target, that one ion channel that is both necessary and sufficient. The thinking that we're starting to develop is that it's not so much a problem with any one ion channel, but rather, with how they are all being regulated. This really comes down to the idea of plasticity and specifically something called homeostatic plasticity. And maybe the magic bullet lies in backing up a step and asking, what has gone wrong with this regulation or with this plasticity that there may be certain molecules involved with that, that has somehow coordinately misregulating different ion channels?
Chris - So Stephanie, do you think you need to go hunting in the genome and ask what is being expressed differently in cells that are now locked into this hyperexcitable state to see if there's a genetic switch that could be thrown to reverse them back to their baseline?
Stephanie - Yes. Well, it's interesting that you would ask this because I was just talking this morning with Steve in saying that I thought we should search for collaborators who want to do this. I do think that there will be something out there to do in order to try to find this kind of upper level control that maybe is what goes wrong and starts changing all their ion channels or start resetting the set point in the abnormal state and try to control that so that we can reset the set point in a normal state.

07:03 - Gene editing and repair kit
Gene editing and repair kit
with Jacob Geim Mikkelsen, Aarhus University, Denmark
Aarhus University Denmark scientist Jacob Giehm Mikkelsen has found a way to use a modified form of HIV - the AIDS virus - to enter cells and quite literally edit mutations out of DNA, replacing them with the correct genetic message. The way that this all-in-one repair kit works is that the team use the virus to bring into cells, proteins called nucleases which goes to the right place in the DNA and cut out the broken gene. Then a strand of genetic material which is included inside the virus is pasted in to make good the repair. Chris Smith has been speaking to him...
Chris Smith has been speaking to him...
Jacob - We have been looking to find new ways to correct mutations within genes. There are some conventional techniques on the market already and they are based on delivering genes that will encode proteins that will take care of the editing within cells. So, what we're trying to achieve here is a safer way by delivering the proteins themselves instead of delivering the gene for the protein. So, what we have been doing is trying to develop viruses to deliver these foreign proteins into cells.
Chris - Which virus are you trying to do that with?
Jacob - In this case, we're using HIV. This is of course a sort of a controversial topic, but what we are trying to do is to deliver specific proteins by using these viruses that will take care of the correction process in the cells that are treated with the virus.
Chris - So, at the same time it's delivering a genetic message in the conventional way of viruses and gene therapy vector, you're also able to exploit the fact that the virus does carry in some proteins as part of the virus and deliver the proteins at the same time.
Jacob - Yes. First off, what we tried to do was just to figure out whether it's possible to deliver these proteins. What we're delivering here is proteins that will then lead to a specific cut in the genome of the cells. But to be able to repair specific mutations inside a cell, people have seen in the past that it's possible to do this by inducing a specific break in the DNA at some specific position that you have predetermined. And then the cell will take care of the repair mechanisms. So, if you, at the same time provide a small piece of DNA that had some homology to that particular region in the genome, then this small piece of DNA will be used as a template for the repair.
Chris - This is rather like a puncture repair kit for a bicycle. You've got a virus particle there which brings in all of the machinery to make good the damage. It cuts open, rather like identifying the hole in the inner tube, and then it's even got the sticky patch inside which is the piece of genetic material you want to paste in, in order to make good the abnormality.
Jacob - Yeah, exactly. You put it in a perfect way. We have called it 'scissors and glue' or 'the scissors and the patch'. So that by providing these proteins that are able to make the cut in the DNA by providing those in the virus, we think we are providing the scissors for cutting in the DNA. At the same time, you can then have these viruses to deliver this little piece of DNA and then this DNA will be able to act as a patch that will make the repair.
Chris - Tell us the nuts and bolts of how this actually works though. Where are the proteins that you're carrying in inside the virus particle and how do you then package up the piece of genetic material that you want to do the repair with?
Jacob - Right. So, that's of course sort of the basics of this - how to get the protein into the virus particles. What we're doing in this case is to fuse this particular protein to the structural proteins of the virus. So, the smart thing about the virus and how the virus is actually working in nature is that it's packaging all its proteins into the virus as one big protein so to speak. It's what is called a polypeptide. So, this polypeptide is packaged into the virus and after budding of the virus from the cell, this polypeptide will be cut into smaller pieces. So, when it does that, this particular foreign protein is released.
Chris - So, that will be floating around inside the virus particle and then when it mix, goes into the cell. It will be released in the cell where it can do its job.
Jacob - That's true and when it gets into the cell, it is now ready to do its action. So, that's what we are seeing. That we actually have quite low amounts of this protein in the cell, but it's far enough to make the action. The action in this case would be to find its position in the genome, make the cut in the genome, and induce this sort of repair.
Chris - What is the evidence that this wonderful strategy does deliver?
Jacob - Yes, so we've done several things. One thing is first, to deliver the protein in the viruses. Not going for repair. Just to see whether this protein has actually able to cut inside the genome. So, we can see what we call 'gene disruption' very efficiently after the delivery of this virus. So, the next thing we did was to then include the patch for repair inside the virus particles so that we do what we call 'all-in-one particles' where we have both the scissors and the patch in the same virus particles. For that, we developed a system where we could screen for specific repair mechanisms. So, in this case, we used a reporter gene, the GFP gene that has a mutation. We are trying to repair this particular mutation. So, we can see when we deliver these virus particles both with the scissors and patch that we have quite significant repair of this particular GFP mutation.
13:33 - Reprogramming pancreatic cells
Reprogramming pancreatic cells
with Qiao Zhou, Harvard
New research is showing that cells in the pancreas, that normally make digestive juices, can be permanently reprogrammed to turn them into insulin, somatostatin, and even glucagon secreting cells, just by exposing them briefly to three reprogramming factors. Harvard's Qiao Zhou spoke to Chris Smith...
Qiao - We are very interested to regenerate insulin secreting beta cells. That's important in the disease diabetes. A few years back, we published a paper and showed quite remarkably, using three factors to convert a pancreatic acinar cells into insulin-secreting beta cells. Now, the acinar cells, their job is to synthesise digestive enzymes. So, we have followed up on these published work.
Chris - What were the factors that you found could do this?
Qiao - The official name, neurogenin 3, Pdx1 and MafA. So, these are the three so-called transcription factors. These factors had been known through years of studies that they are very important in the development of pancreas and beta cells. That has been known. But people don't know that these factors can work together to actually convert an entirely different cell type to beta cells.
Chris - So, it wasn't necessary to initially turn these acinar cells into IPS cells - these induced pluripotent cells and then reprogramme them. You can go from acinar cells directly with just these factors.
Qiao - Exactly. So, that is a very important point you brought up. The standard method nowadays to create some of these endocrine cells is to go through the IPS cells - the pluripotent stem cells and then we differentiate them to these cell types. And this method is basically some kind of - you can view that as short cut, that you can go directly from now acinar cells to this endocrine cell types.
Chris - Do you regard this as a permanent change of fate for these cells? In other words, if you convert these acinar cells in this way and then you keep culturing them or re-implant them into an animal, do they retain that reprogrammed fate indefinitely?
Qiao - That we believe is indeed the case. We have tracked animal models for over 7 to 8 months and when we go back and look at these cells, they're still there and they're still functional. So, we think they have reached the stable state.
Chris - Do you have a feel for how these factors are achieving this reprogramming effect?
Qiao - So, that is a mystery that we're trying to resolve. We don't have all answers yet, but as far as we can tell, these factors, when they enter the cells, it will go via specific places on the DNA importing genes and they can turn on genes that's important for hormone secreting phenotypes and they will shut down these digestive enzymes secreting phenotype.
Chris - And is that without the factors continuing to be expressed? Can you just put them in in a pulse once and then you get this reprogramming effect?
Qiao - Yes. So, that is a very interesting point and that indeed is what we discovered that the factors we put in have a transient expression. They are expressed extremely at very high levels during the first few days. And then their level drops dramatically in about a week. In the end, I think the cells that you made, these hormone secreting cells, no longer have these exogenous factors expressing them. And they reach a stable state.
Chris - What happens if you put these same factors in the same cocktail into a cell that isn't an acinar cell?
Qiao - Very fascinating question and very important one. So, we actually tried this. We made inducible genetic system. Basically, we now can express the same three factors in almost every cell type in the body. We have found the various different cell types. Cells in the liver, various different parts of the body can now express insulin, but are they really beta cells. You could have a cell that's insulin expressing cell, but they may not be a beta cell because they have to have many other properties that are unique to the beta cells. But one place that we study in detail that's quite remarkable is the stomach and the intestine. Some cells in the GI tract can give rise to insulin positive cells and we can secrete insulin. They also have many other properties that look just like beta cells. So, we are very confident at this moment that the stomach and the intestine can also give rise to beta cells or beta-like cells that are remarkably similar to the real beta cells.
18:47 - Worms that regenerate themselves
Worms that regenerate themselves
with Carrie Adler, Stowers Institute
Flatworms called planarians are fated for their exceptional regenerative abilities. But how do the neoblast stem cells that can replace missing or injured organs know, in inverted commas, what to replace. Stowers scientist, Carolyn Adler spoke to Chris Smith. She has found a very clever way to remove discreetly just one organ which is allowed to map the unique gene that drives just the regeneration of a missing pharynx.
Carolyn - We studied these flatworms. For hundreds of years now, people have observed that even tiny fragments of these animals have the ability to regenerate entire animals after they've been amputated. This regenerative capacity depends entirely on the activity of a population of cells that are distributed throughout the animal that are stem cells. These cells are constantly dividing, and can give rise to all of the organs that make up the animal. But the big question that we really wanted to address is how these stem cells kind of know what is missing after an amputation or injury has occurred.
Chris - So, how did you attack that? What did you do to try to understand what the cells do and what the signals are that are driving them?
Carolyn - I developed an assay where it could selectively remove one single organ. We take some animals and I take off the water that they're normally living in and replace it with a concentrated solution containing sodium azide. The initial reaction is, the animals writhe around. They slowly stop moving over the course of 5 to 7 minutes. This large pharynx emerges from the ventral side of the animal. And with gentle pipetting, we can detach the pharynx from the rest of the animal.
Chris - So basically, the animal then has the option to use its stem cells to regenerate it. That's what you're studying.
Carolyn - Yes, so this, what we call chemical amputation leaves a small wound at the border between the pharynx and the intestine. And then the stem cells and the remainder of the animal are left with the task of recognising that this injury has occurred and then regenerating that organ.
Chris - And so, how did you then try to study what the stem cells were doing and what genetic programmes they were running in response to that injury and then the regenerative effort that they went through to replace the missing pharynx?
Carolyn - In order to understand the changes in the remainder of the animal after removal of the pharynx, we first performed expression profiling experiments to identify which genes would be upregulated after removal of the pharynx. We reasoned that these genes would be involved in pharynx regeneration. So then, we knocked down each of these genes one at a time and asked whether the pharynx either could or could not regenerate.
Chris - And so, what's the bottom line? What did you find?
Carolyn - We identified 20 genes that are required for regeneration of the pharynx. Among this population of 20 genes, some genes seem to be required very generally for stem cell function. Other genes, including the forkhead transcription factor FoxA, are required specifically for regeneration of the pharynx, but not for regeneration of any other organs.
Chris - Playing devil's advocate for a moment, what happens if you were to take a pharynx and stitch it onto the animal? So, it still had a pharynx. It was in the wrong place. Would it regenerate another one in the right place? Is there some kind of signal that comes from the missing pharynx that says, "I'm not here anymore" and that's what generates the response, and therefore, would a transplantation fool that response?
Carolyn - That's an excellent question and we think that something like that may be true for these animals. What we observed was that after removal of the pharynx, the expression of this transcription factor, FoxA, increased in the stem cells. What that means is that normally, when the pharynx is present, it could be preventing upregulation of FoxA within the stem cell population.
Chris - So, are you sort of saying that different organs have their own genes which signal, "I want you to make a new pharynx. I want you to make a new intestine. I want you to make a new nervous system" and when you injure a discreet bit of the organism, discreet genetic programmes which is on just to regenerate exclusively that body part?
Carolyn - Our evidence suggests that that may be the case. In our field, there is quite a bit of interest in understanding how the stem cell population is really organised and directs its outputs in response to injury and how that is hierarchically organised at the cell biological level.
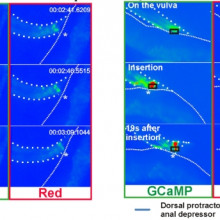
24:19 - Ageing effect on worm reproduction
Ageing effect on worm reproduction
with Louis Rene Garcia, Texas A & M University
As worms age their ability to mate and mate quickly decreases. Luis Rene Garcia has been looking at how growing older affects the reproductive fitness of C. elegans, he spoke to Chris Smith about these findings...
 L. Rene - The goal-oriented task that we were studying is the ability for this little animal - C. elegans (it's a nematode) to inseminate its partner. The animals can do this pretty well, but as they start to advance at age, the first thing that goes wrong is their ability to coordinate all the different little sensory and motor steps that they need to do in order to successfully inseminate their mate. There is such a competition for reproduction that the males have to compete against each other. So, their responses have to be really fast and very coordinated. So, if you were to watch these animals as they age, they look pretty well, but the first thing that happens is, if their coordinations go off a little bit, they fail to compete. And so, their reproductive success starts to decline.
L. Rene - The goal-oriented task that we were studying is the ability for this little animal - C. elegans (it's a nematode) to inseminate its partner. The animals can do this pretty well, but as they start to advance at age, the first thing that goes wrong is their ability to coordinate all the different little sensory and motor steps that they need to do in order to successfully inseminate their mate. There is such a competition for reproduction that the males have to compete against each other. So, their responses have to be really fast and very coordinated. So, if you were to watch these animals as they age, they look pretty well, but the first thing that happens is, if their coordinations go off a little bit, they fail to compete. And so, their reproductive success starts to decline.
Chris - So how did you then take that forward, what were the steps you took to study why they show this progression towards being completely incapable of competing?
L. Rene - We assumed that this is a natural process and the way to get at this, we thought, "Well, let's find a mutant where the animals can mate very well when they're young" but they degrade at a faster rate than wild type. Once we identified that, we can say, "Well, what are the processes that degrade or is different than those animals and those are probably very similar to what normally degrades in a wild type normal male."
Chris - So, how did you go about finding the gene, or a gene, which might be linked to this premature ageing phenomenon that you were searching for?
L. Rene - So, we made the hypothesis that overeating will accelerate the decline of the ability of the males to mate. So then we just surveyed from mutations where visually, the males made more fat and the Sir-2 mutant happened to be a mutant that fit that bill. We then analysed the mating behaviour of these mutants and found that when they're very young, they mated just as well behaviourally were like wild type animals. However, they displayed a premature decline in their mating behaviour. And so then that allowed us to hypothesise that in a wild type animal, this gene is what regulates the durability of the circuit as the animal is ageing.
Chris - What does Sir-2 do? How does it work? And how does it influence this ageing process?
L. Rene - Sir-2 is an enzyme that deacetylates proteins and one of the proteins that it deacetylates are histones. These are proteins that globally regulate RNA synthesis in the genome. But this enzyme could deacetylate other proteins. And so, its job is basically to turn up or turn down the activity of proteins. What we found, this protein regulates the genes involved in breaking down molecules to get energy and genes that are involved in taking those breakdown products and then building up new molecules.
Chris - So, what should genes which are concerned with how I get energy out of food be related to how fast - if I'm a worm that is and maybe it's true for humans as well - why should that be affecting the ageing process?
L. Rene - So, in order for the males to compete with other males, they have to have really fast responses which means that their neurons have to fire very fast and their muscles have to fire and contract very fast. In order for that to happen, the animal has to burn a lot of energy. And so, the animals do have to consume a lot of food. As a by-product of energy consumption, they make this by-product called radicals which can damage the neural circuitry and when it damages the neural circuitry, the neurons fire inappropriately or the muscles contract inappropriately and that messes up their coordination. So, in order to have the fast responses, there's a trade-off. You have to consume more energy, but if you consume more energy then you'll damage the system and then the ability or the length of time that you can have those fast responses decreases. But in the case for the little animals, all they want to do is mate and mate as fast as they can. And so, it's not so important that they can mate for longer period of times, but they mate faster than their brothers. So, faster response is what is selected for.
Chris - And what is the mutant Sir-2 doing in these animals to make them age faster? Why do they accelerate their ageing?
L. Rene - The reason is they make more enzymes that are involved in breaking down glucose. So, they break down glucose a lot faster than wild type animals. They of course, make more ATP. But they're probably making more ATP than they actually need to mate and so, the by-product of making more ATP is making these radicals that are damaging their neurons and muscles faster.
- Previous Tannins: Chemistry in its element
- Next Building the Future










Comments
Add a comment