eLife Episode 57: Malaria and Myrmecophiles
This month, stunning fossil remains of a beetle that evolved to exploit ants and appeared rapidly after ants became social themselves, how inflammation in early life alters the ability of the nervous system to adapt to changing respiratory demands in adulthood, how DNA can be used to track where people picked up malaria, the researchers drawing up new ways to illustrate science, and meet Mike Eisen, eLife's new Editor-in-Chief...
In this episode
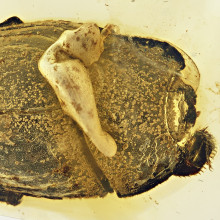
00:34 - First fossil ant-exploiting beetles
First fossil ant-exploiting beetles
Joe Parker, California Institute of Technology
The film Jurassic Park introduced many people to the idea that insects from the era of the dinosaurs occasionally ended up trapped in tree resin that became fossilised amber around them, and preserved them to an incredible degree. The film then took the storyline off into the realms of science fiction, but what researchers can learn from these sorts of specimens is very much science fact, and, speaking to Chris Smith, Joe Parker thinks he’s stumbled upon the 100 million year old remains of a beetle that has a very special relationship with ants...
Joe - What we have here is an imposter of the earliest known ant societies. This is a beetle that targeted the first colonies formed by ants that we know about in the fossil record, and used what we think are chemical and behavioural strategies to infiltrate colonies of these earliest ants, and forge a kind of socially parasitic relationship with them. This is a relationship where the beetle is essentially tricking the ants into gaining acceptance inside their colonies; and once it's inside, it feeds on the resources - in some cases, we think these beetles are being fed mouth-to-mouth by the ants. And so they're essentially these stealth organisms that assimilate into the social fabric of ant colonies for their own selfish needs.
Chris - Because there are examples of this sort of relationship existing today aren't there? There are caterpillars that do this, there are beetles that do this...
Joe - There's about 10,000 species that we know about that use ant colonies as a resource in this way. And these organisms have the amazing name myrmecophiles, which means ant lovers. And across the arthropod tree of life, we see myrmecophiles evolving kind of sporadically. So there are examples of crickets that live inside ant colonies, and trick the ants into feeding them mouth-to-mouth. There are examples, as you said, of butterfly caterpillars - there's a whole family of butterfly caterpillars. But by far the largest number of examples of myrmecophiles are found within the beetles. And what this fossil beetle is an example of is one of the largest groups of these myrmecophile beetles that we know about today. What we found is that, actually, this association between these beetles and ants is evolutionarily very ancient; it really dates very close to the dawn of ant social behaviour. So you can imagine that these beetles have kind of been a constant presence, like a sort of constant part of ant ecology, from the very earliest beginnings of ant colony formation.
Chris - Tell us a bit about the fossil itself, and also how it came to light.
Joe - Burmese amber is an amber deposit from Myanmar. This amber is ninety-nine million years old, so it's kind of slap bang in the middle of the Cretaceous, you know, dinosaurs are walking around. But encapsulated in this amber is this ancient ecosystem with all these different amazing insect species. And I've had the kind of privilege of working with some of the beetles that have come out of this amber deposit. This particular specimen is absolutely incredible because it's immaculately preserved, and is the earliest known definitive example of one of these socially parasitic beetles. And so it has kind of anatomical hallmarks that show that it was specialized for life inside ant colonies. So it's got, for example, defensive modifications of its head and legs so, you know, if ants pick it up and detect it, you know, and try and kill it, it's physically very well defended, it can withdraw its appendages under itself. It's also got what we think are kind of chemical glandular openings on its body and in modern species that we've studied, these chemicals have the effect of manipulating ant behaviour. They produce secretions the ants find very attractive, and have the behaviour manipulating effect of pacifying the ant, preventing the beetle from being attacked. In modern species that we've studied, we also have found that these beetles mimic the ant nest mate pheromones. So ants have chemicals on their bodies that enable them to recognise members of their own colony or intruders, and these beetles mimic those chemicals.
Chris - Well, given that this beetle shows these quite considerable adaptations, it's clearly been evolving for a little while to have become that specialized. So two questions spring to mind. One of them is: when do you think that this symbiotic relationship between this beetle and ants began? And by 99 million years ago, how long had ants actually been around for?
Joe - Well this is the remarkable thing. We think the ants hadn't evolved complex social behaviour much before their first appearance in the fossil record in this Burmese amber; so that 99 million years ago, ants had probably only been social for a few million years. It's kind of just at the dawn of of complex social behaviour in ants. And so it's jaw-dropping that already, by this point, you find these beetles that were clearly living inside colonies of these early ants, and targeting them with the same adaptations for colony infiltration that you see in the modern species today that have this lifestyle. What this tells you is: the evolution of complex ant colonies that are resource-rich environments selected for the rapid evolution of organisms, like these beetles, which had adaptations to infiltrate them. Clearly there's intense selection for organisms to adapt to ant colonies, and these beetles were one of the first groups that were able to kind of turn up at the party. So it evolved very rapidly, you know, in a space of presumably just a few million years; and it's persisted ever since - so, you know, the best part of 100 million years. And what's happened is, as ants have split into modern species, these beetles have kind of hitchhiked along with them. So today there's hundreds of different species of them living with different species of ants in different parts of the world.

06:56 - Brain-altering early-life inflammation
Brain-altering early-life inflammation
Adrianne Huxtable, University of Oregon
Compared with “summer babies”, individuals born in the winter months have a higher risk of a range of different diseases, including neurological and neuropsychiatric conditions. Why though, we don’t know. A tempting theory - but one that’s very hard to test - is that the higher rates of infection that prevail in winter might be to blame. Now, as she explains to Chris Smith, Oregon University’s Adrianne Huxtable has come up with a way to explore how inflammation in early life affects the way at least one part of the nervous system develops and operates…
Adrianne - So what we're showing in this paper is that one bout of sickness early on in life can lead to permanent changes in how the neural circuitry that controls breathing functions into adulthood. We know that early life inflammation is really common, but what we don't know is what lasting effect that might have on this important neural circuitry that controls breathing.
Chris - What's the neural circuitry that you're referring to?
Adrianne - The neural circuitry I'm referring to is housed within the medulla, and the drive for breathing begins here and is then transmitted to relevant respiratory-related motor neuron pools, which innervate the muscles that control your breathing. And we're specifically looking at how these motor neurons that are housed in the spinal cord are influenced by early-life inflammation.
Chris - So if you look at an individual that's been subject to some early life inflammation, or some kind of infection, how does their respiratory effort, or their pattern of respiration, change in line with that past infection or inflammation?
Adrianne - What we're seeing is that it's not necessarily the baseline breathing that's changed. What's changed is the ability of this system to learn, and then respond to subsequent challenges to breathing. So here we're seeing that the system fails to learn properly, so it does not exhibit any plasticity, which could undermine the system in response to disease, or injury, or other challenges to respiratory control.
Chris - How did you do the experiments?
Adrianne - We did the experiments in a rodent model, since most people don't like their central nervous system poked and prodded. Four days after the rats are born we give them an inflammatory challenge; so it's mimicking aspects of a bacterial type infection. Once they're adults, we can then stimulate the respiratory system with changes in oxygen, changes in carbon dioxide, and see how the respiratory system responds. The main stimuli that we're using is repetitive episodes of low oxygen to induce one form of learning within the system. And what we find, is that that one inflammatory stimulus during the neonatal period undermined the ability of the system to learn in adulthood.
Chris - So what should happen in response to that bout of low oxygen exposure in a healthy non-inflamed individual first?
Adrianne - In a healthy, non-inflamed individual, you should see this lasting increase in respiratory motor output. And we don't see that increase if we treated the animals at postnatal day four with an inflammatory challenge.
Chris - Is there anything you can do to reverse this, to try and put them back to the situation that they should be in?
Adrianne - Interestingly, we can reverse it by applying sort of a general anti-inflammatory - sort of analogous to an Advil or a Tylenol. We use ketoprofen in the rodents, and we can reverse one form of learning, but not the other. So we think that there are different pathways to this learning and plasticity, and that they seem to be impaired differently in response to early-life inflammation.
Chris - Where do you think then, based on these observations, that the seat of the problem is? What is being hit during this critical postnatal period, during that bout of inflammation, which leads to this respiratory learning deficit down the road?
Adrianne - I would love to know the answer to that right now. We don't know yet. But we do think that it is related to how different cell types in the central nervous system communicate with each other.
Chris - Presumably it's not the nerve cells themselves? Because the fact that you've got messages being conveyed out of the central nervous system and to the muscles that drive respiration - these animals are aren't stopping breathing, so they must have intact nerve pathways, so that can't be it. So is it something upstream of that?
Adrianne - Yeah, exactly. We don't think it's the neurons themselves that are impaired, and we think it's likely to involve other cell types in the CNS known as glia. We are specifically targeting whether there are changes in microglia, the resident immune cells of the central nervous system, and astrocytes. Astrocytes were largely thought to be support cells within the CNS, but we now know that they can play pretty active roles in how the cells communicate with each other within the CNS.
Chris - And not just for respiratory issues as well, because it strikes me that if one looks at sort of public health data, individuals who are born in the winter characteristically are at risk for a whole raft of conditions compared with people who are not born in the winter. I'm thinking things like schizophrenia. Could it be, then, that actually the phenomenon you've discovered here could be broader than just changes to the way the respiratory networks work; and in fact, you could be finding what underpins why some people are at higher risk when they're born in winter?
Adrianne - Yes, exactly. And things like Sudden Infant Death Syndrome also go up during the winter months. So there may be more here than we know just yet, but it really suggests that there are these really important windows for development of respiratory control; but also more broadly, as you mentioned, neuropsychiatric disorders are also associated with early-life inflammation. There's been a lot of excellent work done recently looking at these different regions of the brain, and trying to understand how different regions are susceptible to early life inflammation. So I think this is a pretty exciting new area of research that might lead us to better understand both some of this acute mortality that you see in infants, as well as some development of things like obstructive sleep apnoea in adults and other disorders as well.

13:45 - Tracking malaria by DNA passport
Tracking malaria by DNA passport
Bryan Greenhouse, University of California San Francisco
Malaria is a scourge in many poorer countries. It can be controlled with interventions like bed nets, spraying and education. But which of these you use depends upon where the malaria is coming from in any given geography. If most of the problem’s an imported one, giving everyone a bed net just wastes money and doesn’t make a dent in the disease. But finding out where most of the cases are coming from has always been very difficult: people give vague histories, the resolution of sampling’s quite low, and even tracking people using mobile phone data is frustrated by international borders. But now, speaking with Chris Smith, Bryan Greenhouse explains how he and his colleagues have a new weapon: the disease’s DNA passport...
Bryan - What we think we've got here is a much better way to understand how malaria moves around in Africa. We're limited right now - in terms of understanding how malaria moves - by basically asking people where they've moved. The problem with asking people is that they may not remember; they may not tell the truth even if they do remember. And even if they do tell us the truth, we may not understand exactly where the parasites that they've been infected with are coming from. So when people come in with malaria, they may or may not know where the mosquito that bit them came from. But the malaria parasites themselves carry a passport, essentially, of everywhere they've been. And that passport is their DNA. So by looking into the DNA of the malaria parasites, we can actually trace where those parasites have come from - whether or not the person who's been infected has any idea where they came from.
Chris - And is that what you did? You actually got the malaria that the person was carrying, and interrogated it genetically?
Bryan - Yeah, it sounds complicated, but actually in terms of what we did in the field it was relatively straightforward. When people get tested for malaria they get a drop of blood taken from their finger usually, and it's put on either a microscopy slide or a rapid diagnostic test. And essentially what we did, for half of this study, was just collect those used cassettes from the test that's already been done, got the parasite DNA off of those cassettes, and then compared them to the DNA from other people that had malaria. And the other half of this study, it was very similar: we just got a drop of blood on a piece of filter paper as part of another study, and extracted the parasite DNA off of that. By doing a DNA fingerprint from these different types of samples we collected, comparing them across hundreds of different samples, we were able to see how malaria was moving around in this part of southern Africa.
Chris - If you've got just the genetic fingerprint of the malaria parasite, how do you actually know geographically where it came from then?
Bryan - That's a great point. The DNA itself is not enough information. You need to know at least some framework of where other parasites’ DNA are coming from. So in this study we had malaria infections coming from something like 30 different health facilities across northern Namibia. And by putting all that information together - where the people came from, and the information that they provided on where they've been - we were able to infer how these parasites are connected to each other and moving around in this part of Africa.
Chris - Are there any other data sources that we can plug into in the modern era? Because you know having travelled across southern Africa myself quite extensively, I was very surprised when I first did that, that I could actually browse the Internet faster than I can at home. Can you trace where people go by where the mobile phones are going?
Bryan - Absolutely. And that's what the state of the art has been up until now, before we started integrating genetics. So the really interesting thing about mobile phones is, every time you turn on your mobile phone,or it connects to a cell tower, that information is recorded by the mobile phone company. As you're walking around with a mobile phone, and you move from one cell tower to another, the mobile phone company actually has information about your movement at some level, in terms of what cell towers are connected to. By taking those data in a very anonymised way, we can get a general sense of how people are moving around. And what we did in this study was: we compared the estimates we had about how malaria was moving from the mobile phone data, to the data that we got from the travel history and genetics. And it was very interesting in that, over some scales - very short scales - we found similar things. So both mobile phone data and the genetic data indicated that a lot of the transmission of malaria was occurring over very short scales - say, a kilometer, the distance a mosquito can fly. But what the mobile phone data missed was that there are also very substantial connections of parasites moving over longer distances within a single country - Namibia, which is the country we focused on most here - as well as between countries. One of the limitations actually of the mobile phone data is that, for the most part, these companies are national; and so it is currently very difficult to trace how people are moving across borders using mobile phone data. And that is one of the most important questions with respect to malaria.
Chris - And if you bring all of this together - I presume, understanding the constraints of one prior measure, the strengths of your new measure - you can begin to understand the disease dynamics a lot more. And that has to be key to trying to control, and ultimately eliminate, the disease from these different geographies.
Bryan - That's absolutely right. We have a limited amount of money to spend on interventions to try to reduce and eliminate malaria. And so we need to be smart about how we put these interventions in place. If malaria is just coming in from somewhere else, but not really spreading locally, we shouldn't really be wasting a lot of money by giving out bed nets, or doing more spraying in the area where the cases are popping up, if they're not actually being transmitted there. On the other hand, if the malaria is spreading quite a bit locally and we have evidence to support that, then we want to be very aggressive in trying to reduce the amount of mosquitoes and trying to eliminate malaria transmission in the area where we're in.
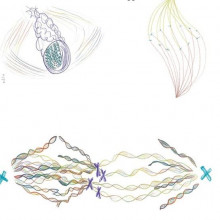
19:37 - Redesigning images in science
Redesigning images in science
Gemma Anderson, James Wakefield & John Dupre, University of Exeter
When students are learning to be scientists, the pictures they learn from are often just a few static images. But in reality, things happen continuously, so still images are always going to miss something. So a group at the University of Exeter decided to get together to represent the process of cell division in a radical new way. Combining the expertise of artist and researcher Gemma Anderson, cell biologist James Wakefield and philosopher of biology John Dupré, a new image was made. An image that shows the whole process of cell division, and not just a few steps. Talking to the team, Adam Murphy heard how the project came to be, beginning with James Wakefield...
James - I never really thought about how I did the science that I did. And as I’ve gone through my 20 years of research, I think it kind of became apparent to me that the default way in which we do science is through this kind of a historical reductionist approach: where you are looking at a live process, something that's going on in the cell - in my case cell division - but you're trying to reduce that down to its component parts. And although that's incredibly useful, there is something missing in terms of what that meant for the cell, if you like. And when I first got to know of John's work, it struck me that the processual view of life was one that had been under-explored in terms of scientific practice, especially in the world of cell biology.
Adam - But how do you create these new images? Artist Gemma Anderson takes me through it.
Gemma - What we're trying to do is to reintroduce drawing into scientific practice, but in a new way, if you like. Because we're not trying to represent scientific objects so much as we're trying to represent scientific processes. So we're trying to think about the process - say, for example, mitosis, or protein folding - as dynamic. We start off with the entire lab; that might be, you know, around six people, postdocs and PhD students and researchers in the lab, and then the P.I. of the lab. So I bring along materials, and questions, and suggestions, and structures that we can use to draw; and then we use the drawing to sort of create a consensus about understanding; then we find new ways of drawing the processes that emerge throughout the session. So they're very productive, they're very collaborative, and they're very helpful to sort of generate new questions for both the scientists and for me as an artist.
Adam - But how hard is it to snap out of your old modes of thinking and look at these images as they were meant to be looked at? James Wakefield.
James - Yes, it is very difficult to do that. And I think that's that's reflected in our publication, in the first two or three of the drawing labs. Both Gemma and I worked incredibly hard to try to release the PhD students and the postdocs from the habit and the learned practices of drawing what you see in terms of things, rather than in terms of processes.
Adam - But philosopher John Dupre seems to think this might not be a bad thing.
John - I think it probably is hard, but I think that may be one of its great virtues: that if people go to the trouble to try and work out why we ended up representing the process the way we did, then it may be really helpful in reflecting on the extent to which they are thinking in an appropriately static ways about the phenomena.
Adam - But what did other academics in the field think of these images? Back to James Wakefield.
James - I think that was one of the one of the most rewarding parts actually of the project. The approach that we took was to show them the final picture first, without any words, without any clues, and without anything to guide them as to what it might represent, apart from saying, “this is a representation of mitosis and cell division”. And so the response that we got from those four individuals was, as I say, incredibly gratifying in one way: in that they were able to really articulate the essence of what we were trying to represent, even though we haven't really told them what it was at all.
Adam - And what are the implications of this work? John Dupre.
John - We hope that it will encourage an awareness that drawing isn't just a way of producing pictures which you could produce much more easily by using some kind of automated technology, but that it’s a way of interacting with phenomena in knowledge-generating ways.
Adam - And finally, Gemma Anderson takes me through the wider implications.
Gemma - When you think about art and science, and how lots of students in school quite often feel a bit torn between, “I like biology and art”. One is more about studying the creative process of, you know, life; and the other is more about engaging in creative processes. And I think what's nice about this project is how we're actually encouraging learning and understanding living processes through the engagement of creative artistic processes, and that seems to work very well.

25:31 - Meet Mike Eisen
Meet Mike Eisen
Mike Eisen, eLife
eLife has a new editor in Chief. He’s called Mike Eisen; he’s a geneticist and he studies flies for a living at UC Berkeley. He also dabbled for a while with being a baseball commentator on the radio, and has been a powerful proponent of open access. I went to see him to find out what he has planned for eLife during his tenure…
Mike - What I want is a world in which I as a scientist, or you as a scientist, or anybody as a scientist who's done something interesting, can communicate that work to us in whatever way they see appropriate. Write a paper. Maybe they'll just share a bunch of figures. Make a video. The format that we use for scientific communication is overly constricted, because we have this notion of a journal that we're stuck with from Francis Bacon. So step one is: scientists should produce whatever it is they want to share with the rest of the community. Step two is: they share it with us in a way that it's completely freely available, everybody can see it, they can access it, there’s no paywall, science is a free and open endeavour.
Chris - So are you saying then, that I go off on some trip somewhere, and I take some photographs, I think there's a scientific story in it; I just write you an essay, and I send that to eLife, and you'd say, “great, we'll publish that”?
Mike - Well I wouldn't say we publish it. I think the whole idea that we are a publisher is the wrong concept. The publisher of science is the scientist. There's a great blog post that someone pointed me to a decade ago or so: on the internet, publishing is not business, publishing is not a process, publishing is not an industry, publishing is a button. That is the way we should think about science communication. I, as a scientist, I do it. If I take pictures of a weird animal, or I do an experiment, or I have a story about how some gene works, you record in whatever way you think is most effective for communicating that with the public, and you publish it. We have a place where you can do it; like, I think we need a central place where people can publish their work - and it's funded by science funders who support the infrastructure just like they do genome databases and other things - you publish your work, and then you come to us, whether it's eLife or some scientific society or another group, and you say to us, “I want you to evaluate my science. Do you think I need to do additional experiments? Have I convinced you that the conclusions I'm drawing from this work are really merited by the experiments and the data I collected, and the context of everything else we already knew about science?”
Chris - How do you give them a gold star if you really like it? What's the eLife badge of honour then?
Mike - So this is the challenge. This is what we're going to be working on now, which is there's parts of peer review that are a dialogue between the authors and the reviewers. That's actually just a dialogue between scientists and other scientists trying to improve their work. The reason why journal title sticks around as such a powerful thing is it actually encodes a lot of information. It tells people what articles to read. There's two million papers published every year and in a future world where you can publish other things, that number is likely to grow exponentially. You can't look at everything that's relevant. You need some guide to know what likely to be interesting to you, and the peer review system in which other scientists read papers and try to render their own judgment about who it's going to be interesting to, is a very useful guide. Right now, that's encoded in journal title. It encodes a sense of how well done the work is, and it encodes a measure of how likely the people who did this work are to do something interesting and important in the future. And that is the primary way in which it's used by, in hiring. And so what I want to do more than anything is for us to figure out: how do we,as not just a publisher or a journal, but as reviewers and editors, how do we communicate the things we're thinking when we read a paper, about who should read this paper, why you should read it, what other things you should know while you're reading it, how do you connect it to the other parts of the literature, and what does it say about the capacity of the people who do it for doing interesting, important, creative science in the future? We want to say that without using this antique system of encoding it in journal title. I like to say, if not for the historical accident - and it is a historical accident that Gutenberg invented the printing press before the internet - led to so many different things in the way we do science publishing, including the use of journal title. We want to shed the historical accident of the birth of journals, in the way that we share the evaluation of scientists. And that's why I came to eLife: because eLife has this fantastic system of peer review, it has an incredible editorial team, it has incredible editors, good at judging works of science; and I want to take that intellectual engine of peer review, and just change the way that they judge works of science. I think if we do that well we can free all of science from these shackles of journal title. In doing so we would empower just a massive flowering of new ideas and new ways of communicating in science, all of which are on people's minds, but have never been able to take off because we are so stuck on journal title.
Related Content
- Previous Meet Mike Eisen
- Next Sensing air pollution










Comments
Add a comment