This week Kat Arney joins us live from the National Cancer Research Institute's conference in Liverpool. We find out how mistakes in cell signalling can cause cancers and why DNA repair pathways offer targets to treat tumours. Also, we explore the latest developments in cancer imaging, including new techniques that allow us to track chemical reactions happening inside the body. In the news, why you need to remove genes to repair nerves, and how clearing out old cells can prevent diseases of old age!
In this episode

01:21 - The National Cancer Research Institute's Cancer Conference
The National Cancer Research Institute's Cancer Conference
Kat Arney offers a roundup of the latest news, and what to expect from the National Cancer Research Institute's annual cancer conference, held this year in Liverpool...
Kat - This is the National UK cancer conference, so it's really big and there's loads of stuff. There are loads of doctors, nurses, scientists, cancer patients. All sorts of people connected with cancer research are coming here today, and over the next couple of days, to talk about the latest developments, find out what's going on and set up collaborations. It's the 10th anniversary of the NCRI, the National Cancer Research Institute, and that's the umbrella organisation for all the cancer funders in the UK. So it's shaping up to be a really big and impressive conference.
 Already we've had some stories coming out of the conference. There's been some news about increasing rates of cervical cancer among young women in their 20s. Now we have a very good screening program for cervical cancer and it prevents many deaths by detecting dodgy cells before they actually become cancerous. And initially, this screening program did cause a drop in cancer rates in young women, but the rates are on the rise again. The majority of cases of cervical cancer are caused by HPV, the human papillomavirus, and this is usually a sexually transmitted infection. So scientists maybe think that a lack of safe sex in young women might be the cause. Be safe out there ladies and help to reduce your cancer risk!
Already we've had some stories coming out of the conference. There's been some news about increasing rates of cervical cancer among young women in their 20s. Now we have a very good screening program for cervical cancer and it prevents many deaths by detecting dodgy cells before they actually become cancerous. And initially, this screening program did cause a drop in cancer rates in young women, but the rates are on the rise again. The majority of cases of cervical cancer are caused by HPV, the human papillomavirus, and this is usually a sexually transmitted infection. So scientists maybe think that a lack of safe sex in young women might be the cause. Be safe out there ladies and help to reduce your cancer risk!
Also, there's a strong focus here at the conference on personalised medicine, and we'll be talking a lot more about that later in the show. This is about testing tumours for specific faulty genes or molecules, and finding out which drugs are most likely to be effective. This is really moving towards treating cancer as a molecular disease, not just a one-size-fits-all thing. So instead of saying, "Well, you have bowel cancer or you have breast cancer..." testing someone's tumour and saying "Ah! You have this, this and this faulty gene, so you need this, this and this drug."
We'll be talking later in the show to Professor Chris Marshall, who has done a lot of work over the past 30 years into cell signalling, and trying to understand how these signals go wrong and how we might target them to treat cancer. He's just done an excellent talk this afternoon all about that.
We've also got a very interesting talk going on about chemoprevention, from John Potter [Prof. John D. Potter, University of Washington, Seattle, USA and Massey University, Wellington, New Zealand]. This is the idea of preventing cancer using drugs. We've been very successful with preventing things like heart disease using statins, but much less successful with cancer. There is some evidence that a breast cancer drug called Tamoxifen, and now new drugs called aromatase inhibitors might help to prevent breast cancer in women at high risk. But there haven't been many other successes like this. So he asks "why aren't we being successful, do we need to completely rethink our ideas about how to prevent cancer?"
So it's looking like it's going to be a week packed with talks, posters, networking events and there's even art exhibitions exploring issues around cancer and how it effects people. It's shaping up to be a fantastic few days, and me and my team from Cancer Research UK are going to be covering all the top stories on our blog, so
check it out to get all the breaking news.
Ben - In some ways, it feels like we're on the cusp of a sea change in attitudes towards cancer, because of personalised medicine, because of gene therapies. It really does seem like we're in a very exciting time.
Kat - It's a really, really exciting time. If you look at when the human genome was sequenced, just a decade ago, it cost so much money - hundreds and hundreds of thousands of pounds - and it took ages. And now we can sequence three cancer genomes in a week for a cost of around one thousand dollars. So we can actually really start to analyse people's tumours, figure out what's gone wrong and work out the right drugs to treat them. So there's a lot of optimism in the field now of cancer researchers and cancer doctors.
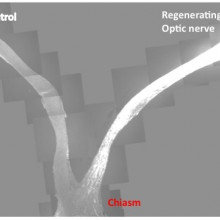
05:10 - Remove Genes to Repair Nerves
Remove Genes to Repair Nerves
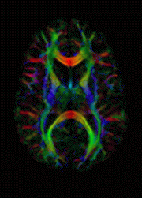
Deletion of two regulatory genes allows damaged neurons to grow back in a sustained way. This could point the way to new drug targets that encourage nerve regrowth.
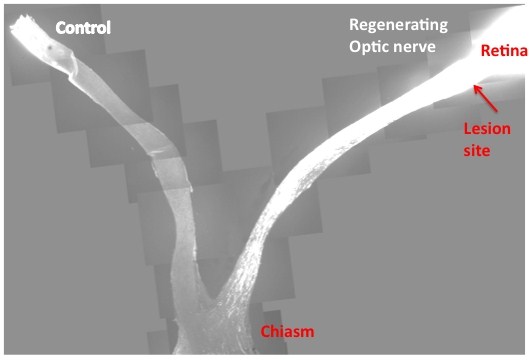 |
| This slide is the composite images from multiple sections that cover both control and injured eyes, optic nerves and the chiasm. In contrast to the control, where no regenerating axons pass the lesion site in the left, PTEN/SOCS3 double deletion allows massive optic nerve regeneration and many axons reach the chiasm. Credit: Fang Sun |
One of the major problems with repairing or re-growing damaged cells from the Central Nervous System (CNS) is the relatively huge distances the cells need to extend in order to meet their target. There are two stages of neuron growth in development: De novo outgrowth and connection in the developing embryo, followed by a long period of "networked growth", where the axons - the long slender projection that carries the electrical signal from one neuron to the next - extend, maintaining their connection at either end. As an animal grows, this second stage can result in axons far longer than anything created by the novel growth in the embryo.
If adult CNS neurons are damaged, however, the de novo methods kick in to attempt a repair. However, this can be slow and unreliable, and doesn't really offer the sustained repair and regrowth required to regain pre-injury performance.
Writing in Nature, Zhigang He from the Children's Hospital Boston and colleagues report that deleting or suppressing two regulatory genes - called PTEN and SOCS3 - enabled adult retinal ganglion cells from the optic nerves of mice to sustainably regenerate a damaged axon.
PTEN, or Phosphatase and tensin homolog, acts as a tumour suppressor, regulating cell growth and replication, while SOCS3, Suppressor of cytokine signaling 3, regulates cell signalling. If you remove either gene on its own, you see a little improvement in neuron regeneration, but deleting both genes together has a remarkable effect, a more than tenfold increase in the number of regenerating cells.
The researchers then looked at the pathways downstream of these genes, and identified a number of factors that were only activated in damaged nerves if these two genes were deleted. This suggests that PTEN and SOCS3 are suppressing growth factors and pathways that promote axon growth, and although each gene acts on different pathways, there is some synergy between them.
Understanding these factors could now provide a target for treatments that would encourage the rapid and healthy regrowth of damaged nerves and achieve functional recovery.

07:37 - Clearing Old Cells Prevents Old Age Diseases
Clearing Old Cells Prevents Old Age Diseases
with Dr Jan van Deursen, Mayo Clinic
Most cell types in our body are being constantly replenished, but we still get old. A sub population of cells are said to undergo senescence - chemical controls kick in and stop them from dividing to produce new cells. When we're young, these are then cleared out by the immune system, but as we age they start to build up in our tissues.
Now, researchers at the Mayo Clinic in Rochester suggest that these cells may play an important role in diseases of old age, as Meera Senthilingam found out when she spoke to Jan van Deursen...
Jan - So we basically set out to test whether senescent cells, which are really cells that accumulate in some mammalian organisms over time were suspect of contributing to age-related disease. So we wanted to test whether that was really the case. All we did essentially was design an animal model in which we could clear these so-called senescent cells. We have two approaches - one approach was to do it lifelong, start at an early age and keep doing it for the rest of the life of the animal and then the other way was to first have the animal age so that the age related disabilities and disorders would be present and then clear the cells, and see whether there would be any beneficial effect of their clearance.
Meera - And whereabouts are these cells actually found? Are they throughout the body?
 Jan - Yeah, they're found in most tissues and organs. They are really thought of as cells that are accumulating as a result of an anti-cancer defence mechanism. So if a cell becomes damaged to a degree that it is likely to develop into a tumour cell, this process of cell senescence is activated. And basically, what it is, it makes these cells stop proliferating as a way to prevent the formation of tumours. The side effect is that you start accumulating these cells in tissues and organs, and they're not just innocent bystanders at that point. This process of senescence really changes the profile of proteins that these cells produce. They start producing and secreting inflammatory cytokines, growth factors, proteases that chew up parts of the surrounding cells. So basically, the secretory components of senescent cells make the life of neighbouring cells much more difficult. So although the senescent cells are relatively small in numbers, maybe a couple of percentage of the total number of cells in a tissue or organ, their negative effects are quite widespread.
Jan - Yeah, they're found in most tissues and organs. They are really thought of as cells that are accumulating as a result of an anti-cancer defence mechanism. So if a cell becomes damaged to a degree that it is likely to develop into a tumour cell, this process of cell senescence is activated. And basically, what it is, it makes these cells stop proliferating as a way to prevent the formation of tumours. The side effect is that you start accumulating these cells in tissues and organs, and they're not just innocent bystanders at that point. This process of senescence really changes the profile of proteins that these cells produce. They start producing and secreting inflammatory cytokines, growth factors, proteases that chew up parts of the surrounding cells. So basically, the secretory components of senescent cells make the life of neighbouring cells much more difficult. So although the senescent cells are relatively small in numbers, maybe a couple of percentage of the total number of cells in a tissue or organ, their negative effects are quite widespread.
Meera - And how did you set about removing these cells?
Jan - We took advantage of the knowledge that tumour suppressor gene p16 is really expressed in most of these senescent cells. It actually is thought to play a role in the conversion of a normal cell to a senescent cell. So we use that to make an artificial gene, kind of a suicide gene driven by that same p16 promoter. So whenever p16 is expressed, we would also express this suicide gene. But the suicide gene is present but it needs to still be activated by a drug. So, senescent cells that express this suicide gene will still not die until we would expose the cells to a synthetic drug.
Meera - And what were your key findings then with regard to the presence of these cells and their effect on particular age-related disorders and which disorders did you see an effect with?
Jan - We needed to test this experimental system and we did that on a so-called progeroid mouse model. This is basically mice that have low amounts of this protein BubR1 and they age about five times faster than a normal mouse. So now the phenotypes in this accelerated ageing mouse that display ageing related disorders are sarcopenia, which is basically muscle wasting, also the loss of fats, both fat deposits in the body and subcutaneous fat, and the subcutaneous fat loss typically gives us the wrinkled skin. The third hallmark of ageing was formation of cataracts which is seen in about 25% of people above 65. So we looked at these three aspects because we know that in those ageing processes the gene p16 was expressed, indicating that in those age-related processes, perhaps p16 positive senescence cells will play a role. We saw that lifelong clearance really can either prevent or delay those age-related disabilities. But when we treat late in life, when those disorders were already present. We could hold them or slow them down from that moment on.
Meera - What are the hoped applications of this then?
Jan - A lot of work needs to be done, but this mouse model can be used to really try to figure out what would be the best strategy of removing these cells. Would that be continuous or would that be once every or once every so often? Would it be advantageous to do it late in life? We agree that lifelong treatments are not really desirable but could also imagine that this is more like a spring cleaning that you do every so often.

13:46 - Drug Addiction, Polluted Cyclones and Roaring Lions
Drug Addiction, Polluted Cyclones and Roaring Lions
with Amir Levine, Columbia University; Amato Evans, Virginia University; Elena Oancea, Brown University; Sarah Klemuk, University of Utah
Nicotine as a gateway to Cocaine Addiction
Being a smoker may increase your chances of also becoming addicted to  cocaine.
cocaine.
Working with experimental animals and also data from human subjects, University of Columbia scientist Amir Levine and his colleagues found that nicotine alters the activity of a gene called FosB, which has previously been linked to addiction. These changes increase the likelihood of developing a subsequent cocaine dependency.
Amir - We saw an increase in the different behavioural paradigms that are related to addiction. Finally, we looked at a certain gene that's called the FosB gene, that has been shown to be very important for addiction and we saw that when we give nicotine first and then cocaine, there is an enhancement in the expression of the FosB gene. And the final step is that we discovered that nicotine basically opens up chromatin and that is how it primes the brain to the effects of cocaine.
---
Cyclone Risk increased by pollution
High levels of pollution are
increasing the intensity of cyclones over Asia.  Carbon-rich brown clouds over the Arabian sea, which have grown six-fold since the 1930s, are cooling the water surface, resulting in a drop in vertical wind shear - the difference in wind speed and direction - between the upper and lower parts of the atmosphere. This increases the efficiency with which storm systems can form, making super-cyclones, with wind speeds exceeding 185 kilometres per hour, much more likely to form. Amato Evan, from the University of Virginia, led the study published this week in Nature.
Carbon-rich brown clouds over the Arabian sea, which have grown six-fold since the 1930s, are cooling the water surface, resulting in a drop in vertical wind shear - the difference in wind speed and direction - between the upper and lower parts of the atmosphere. This increases the efficiency with which storm systems can form, making super-cyclones, with wind speeds exceeding 185 kilometres per hour, much more likely to form. Amato Evan, from the University of Virginia, led the study published this week in Nature.
Amato - What we're really showing is that human activity can actually change this massive atmospheric phenomenon, but what it also says is that because these aerosols reside in the lower part of the atmosphere, if emission stops, this effect would essentially reverse in a timescale of a couple of months. The relevance of these findings is that although we have in this way, changed the climate in such a way that creates very powerful storms, it's not irreversible.
---
Seeing the Light
Skin can 'see the light' to protect us from UV radiation. Elena Oancea and colleagues from Brown University has found that a light- sensitive chemical called rhodopsin, normally found in the retina, is also present in melanocytes, skin cells that produce the suntan pigment melanin. The cells use the rhodopsin to detect UV rays and then switch on melanin production in under an hour. Previously melanin production was thought to occur only after a few days in response to shorter wavelength UVB radiation, which can damage DNA.
sensitive chemical called rhodopsin, normally found in the retina, is also present in melanocytes, skin cells that produce the suntan pigment melanin. The cells use the rhodopsin to detect UV rays and then switch on melanin production in under an hour. Previously melanin production was thought to occur only after a few days in response to shorter wavelength UVB radiation, which can damage DNA.
Elena - So, if a small amount of initial UVA exposure increases the skin's defence to UVB, it's really important not to have for example UVB only sunscreens. The other thing is that if this is a protective response and we have identified the molecules that mediate this response that leads to melanin production then we can activate the pathway artificially and increase the skin protection.
---
New Chemical Elements
Three new chemical elements have been officially named by the general assembly of the International Union of Pure and Applied Physics - IUPAP. Elements 110, 111 and 112 in the periodic table have beennamed darmstatdium, roentgenium and copernecium respectively. They were named by physicists from around the world and are now officially part of the periodic table.
---
The Key to  a Roaring Success
a Roaring Success
The secret of a lion's roar lies in the
shape of its vocal cord, new research has shown. A lion can generate sounds as loud as 114 decibels, equalling that of a jet engine taking off. Scientists had believed that the loud, low-pitched roar was down to the weight and presence of fat within the animals' vocal cords. But now, by analysing samples of lion and tiger vocal tissues, Sarak Klemuk's team at the University of Utah identified the key features to be stretchiness, pliability and square shape of the vocal folds themselves.
Sarak - Little lung pressure is really needed to set these vocal folds into vibration. The Panthera vocal fold is a very square shape. The mechanical properties, along with that square shape allows a lion to generate a very loud roar at a very low pitch.The researchers equated the sound to that of a baby's cry. Both sounds demands attention; but lions use it as a scare tactic to keep intruders away, rather than a call for help...
18:56 - Ras, Raf, Mek, Erk: cell signalling and the future for cancer treatment?
Ras, Raf, Mek, Erk: cell signalling and the future for cancer treatment?
with Professor Chris Marshall from the Institute for Cancer Research in London
Chris - We're now in the stage in our understanding of cancer where we won't be treating a specific cancer such as bowel cancer just because it's bowel cancer. But it will be bowel cancer associated with particular genetic changes and what one person's bowel cancer may have in genetic changes could be different from another's. And what we know now from 30 years or more of research is that many of those genetic changes affect what we call cell signalling. And so, signalling controls when a cell is going to grow, multiply, whether it's going to die or survive or whether it's going to move and these are the three principles at what goes wrong in cancer cells - grow where they shouldn't do, they multiple when they shouldn't, and they survive when they should die.
Kat - And so, what actually are these signals that are going on in cells. We think about signals, maybe like semaphore or Morse code or a mobile phone signal now. How do cells signal?
Chris - Of course, it's molecules which are signalling and what happens is, cells respond as we all do to signal us to do something for a cell to proliferate and multiply it will be a growth factor signal which will drive the cell into cell division. But for that signal to act on the cell, it has to be relayed into the cell's nucleus and it does that by a sort of molecular relay, almost like a relay race where one molecule passes a piece of information to another, and eventually, the message ends up in the nucleus.
Kat - So you've got these molecules that are passing information on. You can see quite easily how that might go wrong in cancer. What sort of things do go wrong to the signalling?
Chris - Well frequently, what happens is that you get mutations in some of these relay components which turn them on all the time. They can't be turned off.
Kat - So you've always got this thing going, "keep going, keep going, and let's keep dividing."
Chris - You've only got an accelerator, and you haven't got a brake. Other types of genetic change, you just remove the brake. So it's like having a car that's parked on a hill and it's got no brake, so it goes to the bottom.
Kat - And also, you presented in your talk today some really nice data about cell movement as well and how faulty cell signalling might make cells go off on the move which is obviously very important when cancer spreads?
Chris - Yes, this is one of the most difficult things to understand in cancer. What is/are the mechanisms which make cells move from their normal place in the body and disseminate somewhere else? And it's that process, metastasis, which is actually the major clinical problem in cancer. And now, by having the methods to molecularly dissect the movement of tumour cells, we're beginning to identify targets of potential drug therapy to stop tumour cells moving.
Kat - Now presumably, this kind of signalling processes that makes cells grow, and make cells move, and make cells die is normally needed for normal health when we need to make new cells if we damage our cells. So, how can we target the processes that are faulty in cancer without switching off the signalling that we need to make new cells when we really need to?
Chris - Well this is a very interesting question and it turns out that many of the genes which are altered in cancer, their primary role is actually in embryonic development. So we're not so dependent on those genes and the proteins they encode for their signalling in adult life.
Kat - So this is when you're developing as a baby in the womb and then they get switched off when you're an adult, you don't need them.
Chris - Yes.
Kat - So then, you should be able to develop drugs that target them when they got switched back on in cancer.
Chris - That's true and the other thing which happens is that some of these proteins, because genetic mutations make them so active, you have a threshold, a window of opportunity where you can inhibit the cancer form, but you're not inhibiting so much, the normal form.
where you can inhibit the cancer form, but you're not inhibiting so much, the normal form.
Kat - So that's when the one that involved in cancer is particularly strange or it has a different shape or something like that.
Chris - Particularly, if it is mutated. If it's particularly active.
Kat - So, where have we got to with developing these kinds of drugs and testing them? Are things available in the clinic now or are things just coming through in the pipeline?
Chris - It's a bit of both. On the pathway which I've spent most of my time working is what we called the Ras Raf Mek Erk pathway.
Kat - Say that again?
Chris - Ras Raf Mek Erk.
Kat - Ras Raf Mek Erk. Lots of abbreviation too.
Chris - The two best targets for drug therapy in that pathway are Raf and Mek, and there's over 20 drugs now in early stage clinical trials that hit Raf and Mek, and one of them, a drug against the BRaf protein which is altered in about half of melanomas. That is now licensed for treatment of cancer patients.
Kat - Is this the drug vemurafenib.
Chris - That is vemurafenib.
Kat - Almost as hard to say as Ras Raf Mek Erk. So we're starting to see these drugs actually getting through trials and to the clinic. Because they're only going to work for a certain proportion of cancer patients, how can we tell who they'll work for?
Chris - We'll have to determine what sort of genetic mutations the patients have in their tumours before we treat them and this now is going to be quite an easy technology. It is not going to be difficult to do this at all.
Kat - You can imagine a situation where you'd take a sample of a patient's tumour, you might test it and see what's gone wrong in it, and then give them a drug or a combination of drugs. Is that how it might work?
Chris - Exactly how it might work. This is what we call personalised or stratified medicine. You get the drug depending on what your mutations are in the tumour. I think these treatments based on these sort of ideas are going to come on-stream very quickly over the next 5 to 10 years.
Kat - So an exciting vision of the future. That's Professor Chris Marshall from the Institute of Cancer Research in London.
25:51 - The Stability of the West Antarctic Ice Sheet - Planet Earth Online
The Stability of the West Antarctic Ice Sheet - Planet Earth Online
with Chris Fogwell, University of Exeter
Ben - How stable is the West Antarctic ice sheet? It's one of the biggest questions in Climate Science. After all, if the ice itself melted then global sea levels could rise by between 3 and 5 metres, and that would be a catastrophe. To work out how stable the ice sheet has been in the past, scientists at the University of Exeter has been using a process known as cosmogenic isotope dating. The technique involves studying isotopes, those are different forms of the same element and Richard Hollingham met up with glacial geologist Chris Fogwell to see what he has found.
Chris F - This is a cosmogenic isotope extraction laboratory and this is one of the first in England. It's basically a clean air laboratory, so dust-free conditions; we use this airlock or antechamber.
Richard - So let's go inside through the sliding door, and you've got a sticky door mat. This is to take dust of our shoes.
Chris F - Yeah, basically dust is our big enemy in these places because it's absolutely loaded with beryllium which is one of the isotopes which we're trying to measure so we try and avoid getting dirt and dust in here at all.
 Richard - And as you might expect the lab itself is sparse, white surfaces, fume cupboards and a blast of air from the vents above us designed to keep the room free of contamination. Here, Chris' team is studying rock samples found on or near the ice sheet.
Richard - And as you might expect the lab itself is sparse, white surfaces, fume cupboards and a blast of air from the vents above us designed to keep the room free of contamination. Here, Chris' team is studying rock samples found on or near the ice sheet.
Chris F - You can kind of visualise the West Antarctic ice sheet as being a giant glacier. It's an ice sheet, so it drowns the topography but it still quarries rocks from the base of the ice sheet and brings them to the surface at certain points. Now, we can pick up those rocks from both up on mountainsides, so where the former ice sheet used to be when it was far bigger and on the modern ice sheet as well which are coming up through the ice. Now, we can test these and look at the long term configuration of the surface of the ice sheet and how it's changed.
Richard - Can we have a look at these rocks then. So, let's wander over to the bench here. This one is quite beautiful. Some sort of granite, is it?
Chris F - It's basically full of quartz and different feldspar minerals. We're principally interested in the quartz because this contains the isotopes that we're analysing here which is beryllium-10 and aluminium-26 and they're produced within the latter structure of the quartz. So, essentially we collect these rocks from different important geomorphological locations across the mountain range and extract the quartz from them. Now once we've got the quartz and we've cleaned the quartz we can extract the minute amounts of isotope from within the quartz.
Richard - What's this isotope, this beryllium isotope?
Chris F - Yes, we have beryllium-10 and aluminium-26. Now these isotopes are only produced by the interaction with cosmic rays which come through the atmosphere. Now they produce the isotope year on year at a reasonably well known rate which allows us to essentially use it as a clock, use the build up of it as an exposure clock since the ice sheet changed its shape or structure.
Richard - So when they're exposed they're absorbing cosmic rays, when they're buried by the ice they're not.
Chris F - Yeah, this is the theory that we base this technique on. Now it's giving us a very good idea for where the upper surface of the ice sheet has been and when it was there, so it can allow us to reconstruct the three dimensional shape of the ice sheet.
Richard - So you got this bit of rock, it's about the size of a house brick. What can that rock tell you?
Chris F - Well it can tell us a few things. Basically, we can get an idea of ice flow direction because we know where the rocks outcrop from, so we know where the rock has been transported from which is one very useful thing. It can give us the structure of the ice sheet back through time, but more importantly, by analysing the concentration of cosmogenic isotopes, it can give us the idea of the time since it's been exposed.
Richard - And what have you found?
Chris F - We've found an interesting pattern which shows that the current configuration of the West Antarctic ice sheet in this sector of Antarctica has remained the same for hundreds of thousands of years. Now, this evidence is based upon the presence of features called moraines on the sides of the mountains. Now above these moraines you get long, long exposure ages, long cosmogenic exposure ages, which suggest that there's been no real glaciation of the mountains, of the upper part of the mountains, for a long period of time. Now, if the West Antarctic ice sheet was to melt then the likelihood is, you would have small mountain glaciers growing as the ice sheet re-grew essentially around its base. We don't see any evidence of that and we use this and the long term preservation of these moraines on the slopes of these mountains as evidence for stability of the West Antarctic ice sheet in this sector.
Ben - Chris Fogwell at the University of Exeter. He was talking to Planet Earth podcast presenter Richard Hollingham and we should say that Chris's work does not definitively prove anything about the stability of the West Antarctic ice sheet. As far as that's concerned, the jury is definitely still out.
31:32 - Imaging Cancer
Imaging Cancer
with Professor Martin Leach, Co-director of the CRUK and EPSRC Cancer Imaging Centre
Ben - The science surrounding cancer is multifaceted. It involves clinicians, chemists, geneticists, and even physicists. The essential work of developing new treatments and clinical approaches is backed up by developments in imaging technology that allow us to detect and observe tumours with ever increasing fidelity. Professor Martin Leach is Co-director of the Cancer Research UK and EPSRC Cancer Imaging Centre at the Institute of Cancer Research, and he joins us now. Martin, thank you very much for joining us. I was wondering first of all, what sorts of roles does imaging play in cancer and what sorts of imaging do you actually do?
Martin - Well, imaging ranges all the way through the cancer pathway. A very important aspect is whether we can detect disease early because the earlier we can detect it, the better we can treat it. Then we have to see what the status of the disease is, how extensive it is, and whether it's spreading through the body because that dictates different types of treatment. And then we need to guide new treatments using imaging, and imaging is very important in a whole range of guidance methods. We do a lot of magnetic resonance imaging (MRI), that's one of the important techniques that's come on the last 20 years or so, but we also do CT and x-ray imaging, and isotope imaging. And all of these have different roles to play and are often complimentary in understanding the disease and how it's behaving.
 Ben - So what are the challenges you're facing in imaging at the moment? What are the goals that you'd really like to reach?
Ben - So what are the challenges you're facing in imaging at the moment? What are the goals that you'd really like to reach?
Martin - Well, we really like to be able to now, not just see where disease is - and that's the historical perspective. If you look at x-rays, you see these spots on the screens, or broken bones, you can see where the problem is, but you don't know much about it. What we really want to know is how the tissue is behaving and then if we treat it, whether that treatment is doing what it's supposed to do. So those are the new challenges.
Ben - So that's almost a bit like comparing an MRI of the brain to an fMRI. The fMRI tells you where the activity is. The MRI just tells you what shape it is and then you're looking to take those steps in cancer.
Martin - That's right, yeah.
Ben - How can you do that?
Martin - Well one of the techniques we've been working on recently allows us to look at the diffusion of water molecules in tissues and this is quite a neat technique particularly from a physics point of view, but with MRI, we can actually measure how far water molecules have moved or diffused in tissues over a period of time. So if you've got a rapidly growing tumour, all of the cells are clustered very closely together and they effectively squeeze all the water out. If you then successfully kill a lot of those cells, you then get spaces in between the cells and a lot more water and therefore, the water molecules can move much further. We can measure that now and this gives us a way of picking up tumours, detecting them, but also, it helps us to see how far they've spread throughout the body. So we're working now on top of that to try and develop computer methods to measure how much disease there is in the body and then when we've got new treatments that are hoping to reverse the spread of disease, we should be able to detect whether that's working or not.
Ben - So you essentially take a baseline for a patient and then you'd know how the water travels. Either, I guess you could compare healthy tissue with unhealthy tissue or you just know how it started out and then months down the line, you can see how it responds.
 Martin - That's right, yeah. Usually, we do baseline scans and then do follow up scans so we can look in the same tumour.
Martin - That's right, yeah. Usually, we do baseline scans and then do follow up scans so we can look in the same tumour.
Ben - You've mentioned the very interesting physics about this but clearly, computer scientists also have a very large role to play in how we actually understand and interpret that information.
Martin - Yeah. A large part of our work is developing analysis methods to analyse and present the data in a simple way. So this is giving us new tools. In fact, to look at some of the heterogeneity in tumours so that's the fact that some of the tumour cells are driven by one pathway and then perhaps some of the cells have adapted to be driven by a different pathway, and we can begin to try and pick up those differences with a range of imaging techniques.
Ben - So this is really looking at the chemistry, not just looking at the structure, but actually seeing what chemical reactions are taking place. I guess that also will help you to lead and advise clinicians on what treatments might be appropriate.
Martin - Well that's what we're aiming for. In this year of personalised medicine that was discussed earlier, we want to be able to monitor individual treatments, identify what may be the most appropriate treatment and particularly identify if that treatment isn't working any longer and we need to switch. So that's an important area and chemical techniques are central to that. So we've had positron emission tomography for quite a long time and that allows us to probe some important pathways in the cells, but the problem with that is that we can't tell how the molecule we're looking at, and it's a probe molecule, has changed. Cells of course are all about interacting with molecules and making them do different things. So we're now working on a new technique called Dynamic Nuclear Polarisation (DNP) and this essentially is going to make MRI much more sensitive and allow us to look at very small levels of molecules. So, with MRI, we normally look at water and we see only about 7 molecules in every million. So if you thought about the UK population of 60 million, we would be seeing about 400 people. With DNP, we can take that up to about 10 million people. So, that gives us the possibility of looking at molecules that are only present in relatively small amounts and one of those that we're looking at is pyruvate. That's a very important intermediate in the energy supply to the cell, and is very much influenced by cancer. So we're already finding, looking at some of the new treatments that have been developed at the Institute of Cancer Research that we can see changes in that metabolism at a very early stage in the application of the new treatment. And we hope that is then going to inform how we can look at those patients in early stage trials to test whether these new treatments are really working.
Ben - How do you go about taking an idea from the incredible physics that we have and then - you at the ICR, make sure that these things work, and that we know that we can see it - how does it then get into being used in medicine? Surely, all of the radiologists or whoever's role it is in the hospitals need to understand the new techniques in order to interpret them.
Martin - Yeah, well we go through a range of tests first before we try and take these techniques through to the clinic, but then what we try and do is bolt them on to early stage studies, or to routine studies that the patients are having where we can just add this on. It depends on the type of measurement we're doing, but we've introduced a number of these approaches successfully into early stage trials of new treatments. And that gives us feedback on whether they are actually useful imaging techniques and if they are, we then try and move to generalise those to other centres and that helps more clinicians understand how useful they are and gives us more evidence. So, we go through a series of trials and then clinicians will learn about them from conferences and the papers that are published.
Ben - Well thank you very much. That's Professor Martin Leach from the CRUK and EPSRC Cancer Imaging Centre.
39:35 - New Targets for Cancer Drugs
New Targets for Cancer Drugs
with Madhusudan Srinivasan, University of Nottingham
Kat - I'm joined by Madhusudan Srinivasan. He's Clinical Associate Professor in Medical Oncology at the University of Nottingham and he'll be presenting a paper at the NCRI Conference this week about a new target for cancer drugs, and that's blocking the DNA repair mechanism. Good evening. Thanks for coming on the show.
Madhusudan - Thanks, Kat.
Kat - So let's start by going a bit back to basics, why do cells need to repair damage to their DNA? How do they get damaged and why do they repair?
Madhusudan - Well that's a very important question, Kat. If you look at a normal cell, we all know DNA is the building block of life. Sequencing the DNA makes genes. Genes make proteins, but the problem is, in normal cells, the cells are constantly producing damaging agents. These are called oxygen free radicals. These free radicals can actually damage the DNA. If they damage their DNA, the genes can get corrupted and that can make abnormal proteins. In order to avoid this situation, what normal cells do is they're constantly scanning the DNA. We call this scanning the genome to maintain stability of the DNA and DNA repair pathways are critical for maintaining this genomic stability.
normal cell, we all know DNA is the building block of life. Sequencing the DNA makes genes. Genes make proteins, but the problem is, in normal cells, the cells are constantly producing damaging agents. These are called oxygen free radicals. These free radicals can actually damage the DNA. If they damage their DNA, the genes can get corrupted and that can make abnormal proteins. In order to avoid this situation, what normal cells do is they're constantly scanning the DNA. We call this scanning the genome to maintain stability of the DNA and DNA repair pathways are critical for maintaining this genomic stability.
Kat - So there's damage to DNA created by just the processes going on in cells, but presumably also from other things like cigarette smoke or ultraviolet light from the sun, things that we know damage DNA and cause cancer.
Madhusudan - Absolutely, right. So what I spoke to you about was endogenous DNA damaging agents and you have exogenous DNA damaging agents, and the most important ones are environmental agents - smoking is a main culprit. There are environmental toxins such as lead, industrial toxins. Now these hydrocarbons as we call them can actually directly damage DNA. And when the damage to DNA happens, the bases get corrupted and that leads to what's called mutations and it is the mutations that drive the cancerous phenotype.
Kat - So we've got this DNA repair going on, that when we get damaged then we can get cancer. But also, what's gone wrong with the DNA repair in cancer cells because DNA repair is also a bit messed up in some types of cancer, isn't it?
Madhusudan - That's right. In fact, the relationship between DNA repair and cancer is quite complex. On the one hand, suboptimal DNA repair can actually increase the risk of cancer. It can lead to a cancerous phenotype. On the other hand, certain tumours actually upregulate DNA repair and when they upregulate DNA repair, what they do is it gives them a selective survival advantage and it also makes them resistant to certain treatments that we use in the clinic.
Kat - So things like radiotherapy that damage DNA and cancer cells and make them die, they would become resistant to that kind of treatment?
Madhusudan - That's correct. It's radiation therapy and also, several chemotherapeutic agents that we use in the clinic.
Kat - So, some of the drugs that you would treat them, they actually become resistant because they're repairing the damage that's being aimed at them to kill them.
Madhusudan - Absolutely, right. Yeah.
Kat - So, talk a little bit about your research. So you're studying a particular repair pathway in cancer cells and in healthy cells as well. Tell me a little bit about that and what's going on with that.
Madhusudan - Okay, so mammalian cells that's human cancer cells have several DNA repair pathways. We know there are at least 6 different DNA repair pathways in man. One of the DNA repair in that is called base excision repair and this particular pathway is absolutely essential for maintaining the correct bases in your DNA.
Kat - So the letters. The specific letters of the instructions.
Madhusudan - Absolutely. So they are absolutely essential to maintain the letter, keep the script going. Okay, so that's the base excision repair. Now this base excision repair was discovered about 20 to 30 years ago and over the last decade or so, we know a lot about base excision repair - what are proteins involved in based excision repair, how the process is coordinated. One of the proteins that my lab has focused on is a protein called human AP endonuclease. It's also called APE1. This particular protein is very essential for base excision repair and the work in our laboratory, which has been going on for the past five to six years, is trying to understand what this protein does in normal cells, what this protein does in cancer cells, and how can we exploit them for cancer treatment in patients.
Kat - So here at the conference, you're presenting some really exciting data where you've been trying to block this particular protein that's involved in this DNA repair and what do you see when you block this?
Madhusudan - What we are trying to do in the laboratory is first understand what's happening to this particular protein in tumours and what we see is APE1 is frequently over expressed in tumours.
Kat - So it's working too hard.
Madhusudan - And also, the tumour cells are over expressing it so that they can try and circumvent other damages and continue to survive and lead to resistance to treatments. So that's one thing that we've understood from studies in human tumours. Then what we've gone on to do is, now that we know that this protein is very essential for cancer cells, we've actually established a drug discovery program to try and block this particular protein. So we isolated what's called a small molecule inhibitors of APE1.
Kat - These are little tiny drugs.
Madhusudan - Little tiny drugs, compounds to be precise that block the functioning of APE1. The next stage in our research has been to try and exploit novel treatment strategies to try and define which group of patients would actually benefit from APE1 inhibitors. And that's what we are presenting tomorrow and I'm really excited about that because this new field in cancer called stratified medicine and personalised cancer medicine, where we can actually look at tumour biology and then tell our patients in the clinic that this is the treatment that you're going to have.
Kat, one of the problems that I face in the clinic when I see a patient is, before I start the treatment, there is no way in which I could know whether chemotherapy is going to work or radiotherapy is going to work or not. For the first time in the last three years, we actually have tools where we can actually predict who might be able to respond to treatment, and who may not be able to respond to treatment. This is in its very early phase and over the next 5 to 10 years, we are going to see a huge explosion in this personalised cancer treatment. And the research that we are presenting tomorrow is exactly in personalised cancer treatment approach where looking at tumour biology, we can actually target our APE1 inhibitors for treatment.
Kat - So what particular types of patients or types of cancers might these drugs be suitable for?
Madhusudan - So when you look at personalised cancer treatment, we believe that APE1 inhibitors are particularly going to be beneficial in breast cancer, in ovarian cancer, in pancreatic cancers. A proportion of these tumours, up to 10 to 20% of these tumours, are deficient in a particular gene called the BRCA genes. And what we know is, if we can identify those tumours, studies in our laboratories have suggested that APE1 inhibitors are likely to be particularly sensitive to those tumours.
Kat - So you could do a test for a person with that type of cancer and say, "Okay, you have a fault in this BRCA gene and then you might benefit from this type of therapy." How close are we to actually testing these drugs in clinical trials?
Madhusudan - That's a very, very important question and as you know, drug discovery is a long drawn expensive process and the stage we are at the moment is what's called a hit to lead conversion.
Kat - So it's very early still.
Madhusudan - It's very early, but potentially very exciting. So, I think our medium term to long term strategy is usually get a pharmaceutical partner and try and drive this research program for patient benefit.
Kat - So at the moment this is just still in the lab. You're trying to find the best kind of things to take forward to develop into a drug.
Madhusudan - We're fine-tuning the structures of the compounds so that we can decide which one to take forward, so you're quite right.
How does one get into cancer research?
Martin - I think you need to follow your interest to start off with. Of course, there's a very big route in from biology into cancer because of the basis of cancer, but there are many other areas that are involved in developing new ways to deal with cancer whether it's physics, chemistry - where we're developing new drugs, engineering in terms of equipment and a lot of computer scientists. There's a lot of epidemiology in cancer of course. So, there are a very wide number of routes, but usually, you need to go through university training at some level and then there are a lot of different possible routes from then on.
Ben - Kat, with your CRUK hat on, you must deal with an awful lot of people from a lot of different backgrounds.
Kat - Absolutely, yes. Cancer Research UK funds statisticians and epidemiologists - those are people who do very large population studies to find out what causes cancer. We have people who are involved in designing clinical trials. We have chemists who are designing potential new drugs. All sorts of things, physicists, people doing weird computing stuff, all kinds of people are involved in the effort to beat cancer and do different types of cancer research. That's not just about biologists or doctors. There is a huge range of people and also, nurses as well. Research nurses are really important for helping to run clinical trials. So there's a route there through nursing and through the medical profession.
What are the different stages of Cancer?
Kat - There are lots and lots of different types of cancer. People say the word "cancer" but it's actually many, many different types of disease and as we've heard today, they're all very different.
It depends what genes have gone wrong in them, it depends what type of tissue in the body they started in, and doctors tend to stage cancer according to how far it's spread.
Normally, a cancer will start when a cell starts multiplying out of control. We think about cancers not just as a little clump of cells that have gone wrong, but really as a rogue organ - so it's starting to grow a blood supply, and it's starting to involve immune cells and cells from the tissue around it - that's kind of very early stage cancer. And if you can catch cancer at that kind of stage, it is very easy to remove with an operation and that the person will get better again. This is why it's so important to try and catch cancer early, to go to the doctor if there's something wrong, if it doesn't feel right, because in many cases, early stage cancer can be successfully cured with surgery. Then you start to look at the later stage, when it starts to spread. It spreads through the lymph nodes in the body or starts to spread through the bloodstream. And so, that can be really difficult to treat.

51:45 - Should we wear sunglasses?
Should we wear sunglasses?
We posed this question to Dr. Mike Cox, Senior Lecturer in Clinical Vision Science, University of Bradford... Mike - We need to think about the light reaching your retina. To get there, it has to travel both through the sunglass lens and through your eye's pupil. In a 20-year-old person, on a bright day, the pupil diameter will be around 3 mm. With a dark pair of sunglasses on, this will make the pupil dilate to a diameter of around 4.5 mm. That means roughly, 2.5 times more of the light reaching the outside of your eye can reach the retina by travelling through your pupil. But don't forget, due to the sunglass lens, only around a tenth as much light is able to reach the outside of your eye and travel through the pupil in the first place. The total amount of light reaching the retina is reduced by a factor of around 4. So sunglasses really do work, even though your pupil will dilate if you wear them. Thinking about eye safety with your sunglasses, too much invisible ultraviolet light can harm the retina inside your eye. If a sunglass lens doesn't absorb ultraviolet light, but those absorb visible light then with the sunglasses on, the pupil dilates and the ultraviolet light can still travel through the lens, reach the outside of your eye, and head through the pupil. As the pupil is bigger, more of this ultraviolet light reaches the retina inside your eye and then over a long time, this could harm your eye. Fortunately, if you buy sunglasses in Europe, they should have the letters C and E marked on them. If you see the CE mark then the sunglasses will give you the proper protection against ultraviolet light. If you don't see the CE mark, don't buy or use the sunglasses.
Diana - So the pupil will dilate to allow in more light, but not enough to counteract the effect of the sunglasses. Further, a good quality pair of sunglasses will filter out UV light which is a really damaging stuff. And top marks to Geeza and Diver John on the forum who said pretty much that.
- Previous Repairing Nerves and Roaring Lions
- Next Why Are Planets Round?










Comments
Add a comment