Is standing or reclining best for the perfect suntan? Can we see atoms? Why add pennies to Big Ben's pendulum? It's a question and answer show so we shoulder your scientific conundra! We'll find out how web companies keep up with growing data demands, what causes white ridges on fingernails, and why a clean glass keeps cola fizzier. Plus, in Kitchen Science, we find out how to balance a broom whilst blindfolded!
In this episode

01:34 - Climate change has affected crop yields
Climate change has affected crop yields
This week researchers from Stanford University have revealed that, in the last 30 years, corn and wheat crop yields have dropped by 3.8 and 5.5 per cent in response to climate change. This decrease in production has occurred in spite of technological advances, pest control measures and the use of fertilisers...
Publishing in Science, David Lobell and his team note that all major growing regions for wheat, maize, soybeans and rice have experienced increases in temperature since 1980. The notable exception was the US, which experienced cooling. And, although corn and wheat have seen a drop, soybean and rice crops have remained roughly unaffected across the globe.
To reach their conclusions, the researchers developed two models: one which mimicked the observed increases in climate temperatures; and one which assumed temperatures had stayed the same since 1980. They also used statistical tests to see if precipitation changes affected crop yields. The results demonstrated that increases in temperature had reduced global yields of wheat by 5.5 per cent, and increased rainfall also had a negative effect on crops but it was much less marked than temperature.
Given that the U.S. is a notable exception, it shows that analysing climate change data on a country-by-country scale will be misleading. This team have shown that temperatures in the United States haven't increased in a statistically significant way, and subsequently their crops haven't suffered. But the rest of the world has seen a very obvious and worrying change. And this doesn't mean the U.S. is unaffected, since decreases in food availability across the world will inevitably have an effect on the cost of imports. In fact, the authors argue that it already has.
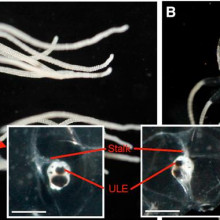
04:10 - Box Clever: jellyfish use eyes to navigate
Box Clever: jellyfish use eyes to navigate
At just one centimetre across, the box jellyfish - Tripedalia cystophora for lovers of Latin - makes a poor contender for being a paragon of intellect. But now  new research has show that these tiny creatures can actually navigate by following the outlines of objects in the sky above them.
new research has show that these tiny creatures can actually navigate by following the outlines of objects in the sky above them.
The jellyfish live in-shore among the roots of mangrove trees where they prey upon tiny light-loving plankton species that gather in sunny spots along the margins of the swampy surroundings. But if they wander too far from the shoreline and away from their food source, the jellyfish risk starvation; consequently they are rarely found out in open water.
So how do they avoid being swept away?
University of Copenhagen scientist Anders Garm and his colleagues suspected that the jellyfish might be making use of the 24 "eyes" that adorn structures called rhopalia on each of the four sides of the animal's box-shaped body. Surprisingly, two of the eyes in each rhopalium, one looking up and one looking down, actually use a lens system similar to our own to focus light.
To find out what this upward-gazing eye might be capable of seeing, the researchers used a wide-angle lens to take photographs from just below the water surface. The pictures reveal that, owing to the way that water causes light to bend or refract, each eye would be capable of surveying a 180 degree field of view. And from distances of up to 8 metres away, the outline of the mangrove canopy against the sky would be clearly visible to the jellyfish, providing them with fixed reference point to keep them close to home.
Working in a mangrove swamp in Puerto Rico, the researchers then tested the theory by placing locally-caught box jellyfish into a floating, circular open-toppped tank that was partially submerged in the seawater. This meant that the animals could see the sky, and the overlying trees, but were isolated from any water-borne chemical cues that might provide navigational aids.
The researchers then slowly moved the tank different distances away from the shore and watched the movements of the jellyfish. Incredibly, at distances up to 8 metres, they all swam towards the shore-facing side of the tank. At distances greater than 8 metres, the animals moved randomly again, consistent with having lost the visual reference of the treeline, just as the underwater photographs had predicted.
The team don't know exactly how this intelligent photaxic behaviour is achieved but they speculate that it could be a neurological adaptation of a solar compass. The animals actually lack brains, although each rhopalium carries an associated nerve ganglion equipped with 1000 neurones, and a "ring nerve" links the four ganglia, presumably helping to coordinate the animal's response to what its eyes are seeing.
But regardless of how it works, as the team point out, their observations "defeat the idea that a central brain is a prerequisite for advanced behaviour."

07:31 - Antihydrogen trapping
Antihydrogen trapping
Every particle we know of has an anti-particle, so there are anti-electrons, anti-protons, anti-neutrons etc. These seem to behave pretty much the same as their normal relations, but they have the opposite charge, and if an anti-electron meets an electron they anihilate completely releasing a huge amount of energy in the form of photons.
 Physicists don't understand why there is far more matter in the universe than anti-matter, so they would like to study anti-matter to understand it. The problem is that the only way to make it is in violent collisions so it is enevitably moving very very fast, so you don't get a lot of time to study it, and whilst you can slow down charged particles magnetically, if you want to study the details of anti-atoms slowing them down is much more difficult
Physicists don't understand why there is far more matter in the universe than anti-matter, so they would like to study anti-matter to understand it. The problem is that the only way to make it is in violent collisions so it is enevitably moving very very fast, so you don't get a lot of time to study it, and whilst you can slow down charged particles magnetically, if you want to study the details of anti-atoms slowing them down is much more difficult
In November researchers at CERN managed to trap 38 atoms of antihydrogen for a maximum about 0.2 seconds. Now they have managed to trap over 300 antihydrogen atoms for a maximum of 1000s or about 17 minutes.
They are now moving onto studying the anti-hydrogen, looking very carefully at the energy levels in the atoms, and possibly even finding out whether anti-matter is attracted or repelled gravitationally by ordinary matter

09:46 - The Brain's First Whiff of Nicotine
The Brain's First Whiff of Nicotine
with Professor Daniel McGehee, University of Chicago
Chris - A new study sheds more light on how the brain responds to its first ever whiff of nicotine. By looking at activity in the brain tissue of a rat as it's exposed to nicotine for the very first time, Professor Daniel McGehee, at the University of Chicago, has found that the drug triggers changes in the brain's circuitry, which makes people much more likely to then get hooked.
Daniel - The dopamine system is this reward pathway. The effect of addictive drugs on behaviour is dependent on a change in dopamine signalling, so that's been a focus for our work and many other groups. We know that dopamine activates a specific type of receptor, a protein that's on the surface of many different neurones. And these receptors are excitatory - they depolarize the cell, causing it to fire more so more electrical activity occurs - and we know that that's the first step in the process. What our most recent study has shown is that this actually initiates a whole cascade of events. One of the things that happens is that the inputs that normally excite these dopamine cells become stronger. The synaptic connections - the points of connections between cells - are stronger after nicotine exposure.
Chris - So are you saying that the nicotine comes in, it stimulates some dopamine-producing nerve cells to make more dopamine, which the brain experiences as being a pleasurable experience, and at the same time, the cells that make that dopamine, which are connected to other nerve cells, begin in future to respond more strongly to those connections than they would have done before?
 Daniel - That's a nice description of what our data and others have shown, yes. The connections are stronger and this is something that persists for days. What we're looking at is the steps that lead to that change. Even after the nicotine is gone, these connections remain strong for between five and ten days after exposure, and just to emphasise, that's a change in an animal that has not seen the drug before and it's in response to one exposure.
Daniel - That's a nice description of what our data and others have shown, yes. The connections are stronger and this is something that persists for days. What we're looking at is the steps that lead to that change. Even after the nicotine is gone, these connections remain strong for between five and ten days after exposure, and just to emphasise, that's a change in an animal that has not seen the drug before and it's in response to one exposure.
Chris - And when the person has had that change happen in their brain, what then makes the person keep reaching for the cigarette packet? Because if their cells are now producing more dopamine because the connections to them are stronger - the cells are getting more excitable than they were before - then why carry on smoking?
Daniel - Yes, that's a great question, and one that we would love to have a complete answer to. The transition from occasional use or a single exposure to the full-blown addicted smoker is a long and complex process that we really don't understand. We know that there are huge adaptations happening in the brain areas that we're looking at, but it's also very likely that other brain areas play critical roles. In adolescents who are experimenting with smoking, they will quite often sample cigarettes at a very low rate, like once a month, once every two weeks etc. One theory that I like is the idea that the duration that this increase in excitation in the pathway persists for is contributing to that process and establishes a framework for associating the tobacco and nicotine with pleasure, and that basically sets the stage for the progression to full-blown addiction. The progression is going to be involving this process but also almost certainly other processes that we don't yet understand.
Chris - Does it transfer to other drugs? Because if you rewire this circuitry which makes your brain much more prone to getting hooked on things, and then another drug comes along, does the tobacco pre-priming mean that some cocaine is much easier to get hooked on that it would be without this happening?
Daniel - That's certainly a possibility. It's beyond the scope of what we're looking at right now, but co-use of a variety of different substances is quite common, so the idea that exposure to one drug modifies or enhances the rewarding effects of another drug makes perfect sense to me, as far as I understand the system, and this could be one of the ways in which that could happen.
Chris - I think 75% of smokers say they would like to quit - that's the intended quit rate - though the successful quit rate is another matter. What about using this information to help people to quit or to not get hooked in the first place?
Daniel - I do think that this adds to the public health message that I think is being broadcast quite effectively in many parts of the world - that exposure to tobacco products and nicotine is potentially addictive and long-term exposure has hugely negative health consequences, and there's just no argument here. Even occasional and casual use of tobacco products could be inducing persistent effects on parts of the brain that we know are involved with reward and addiction. As far as the potential for treatment of the full-blown smoker who is trying to quit, we certainly hope that identifying steps in the process, in terms of cellular events and molecular events that are happening inside neurones, may lead to the identification of new drugs that will improve the abysmal quit rate that's out there. I do think that the most important message is that there are changes happening with the very earliest exposures, and they are part of the progression to full addiction.
Chris - Well they say that it only takes one cigarette to get you hooked, and that evidence does look compelling. That was Professor Daniel McGehee, from the University of Chicago - he's published that work in the Journal of Neuroscience this week.

Using antioxidants to combat radiation poisoning
This week, researchers from The University of Pittsburgh have found that a chemical similar to that found in red wine can protect against radiation sickness.
 Specifically, they looked at gamma (γ) radiation and how its effects might be reduced by a substance similar to resveratrol. Resveratrol is an antioxidant commonly found in wines, grapes and nuts; and plants commonly use it to fight off bacterial or fungal infections. The reason the researchers think that an antioxidant might help in protecting against radiation exposure is that they 'mop-up' the free radicals that gamma radiation can produce. And it's these ionised free radicals which do the cell damage.
Specifically, they looked at gamma (γ) radiation and how its effects might be reduced by a substance similar to resveratrol. Resveratrol is an antioxidant commonly found in wines, grapes and nuts; and plants commonly use it to fight off bacterial or fungal infections. The reason the researchers think that an antioxidant might help in protecting against radiation exposure is that they 'mop-up' the free radicals that gamma radiation can produce. And it's these ionised free radicals which do the cell damage.
Publishing in ACS Medicinal Chemistry Letters, Michael Epperly and colleagues first tested the naturally-occurring resveratrol on live cells in flasks, which were exposed to radiation. They found that these cells were given some protection by the chemical but, when they tested it on a mouse, it had very little positive effect. So the researchers turned to another, similar chemical known as acetyl-resveratrol. This time, the drug produced an eighty per cent survival rate amongst the mice. The difference between the two drugs efficacies is most likely because acetyl-resveratrol is more slowly metabolised and therefore lasts longer to provide its protective effects.
And the authors also argue that this could be good news for cancer patients, since the other candidates for anti-radiation drugs work by suppressing cell apoptosis (that is, cell death) and could therefore aggravate a cancer. Because they think acetyl-resveratrol is using a different mechanism, it could be a better alternative and, they add, it is relatively cheap to produce.

17:57 - Space Dragging Confirmed by Gravity Probe B
Space Dragging Confirmed by Gravity Probe B
In 1918 two Austrian physicists worked out that if Einstein was right any large spinning object should drag space and time around it. So if you are near the spinning object your idea of not spinning should be different to the idea of someone in deep space.
The problem is that although this would be quite noticeable if you were near to something very heavy spinning very fast, there is nothing nearby that is heavy enough to make a large effect. Near the Earth the effect should only be tiny.
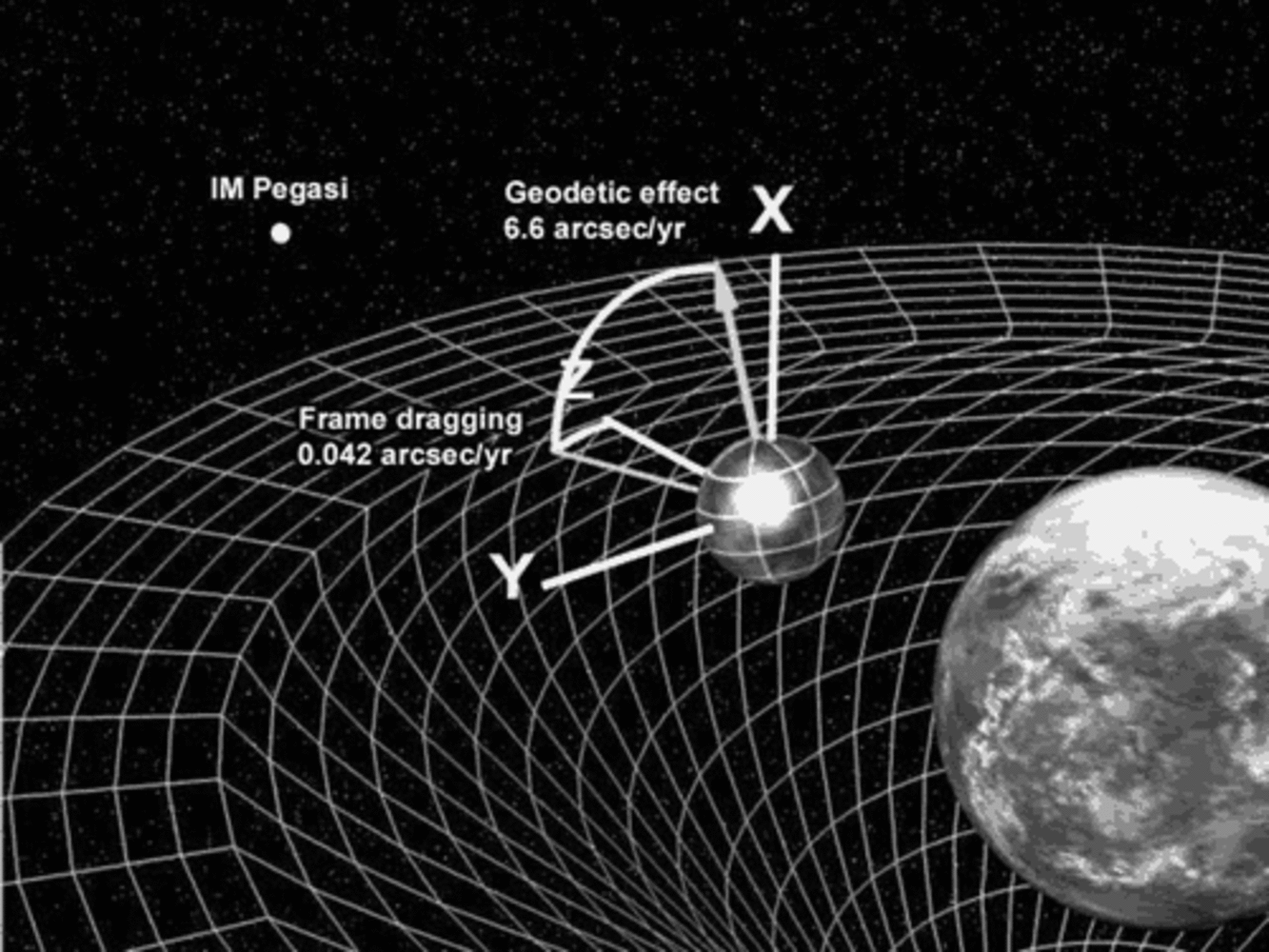 This is such a big problem, in fact, that the project to measure this effect has taken 52 years, and produced a satellite called Gravity probe B. This is an incredible mission using some of the most outstanding physics out there.
This is such a big problem, in fact, that the project to measure this effect has taken 52 years, and produced a satellite called Gravity probe B. This is an incredible mission using some of the most outstanding physics out there.
Basically, it involves incredibly smooth spheres covered with a thin layer of superconductor spinning very fast and acting as a gyroscope.
If there are no external influences, these should keep pointing in the same direction. The satellite detects their direction using superconducting sensors called SQUIDs and compares them to a fixed star. The whole thing has to be cooled to make sure the superconductors work, and minimise thermal noise; to reduce friction, it is cooled using superfluid liquid helium, which has no viscosity, minimising its effect on the gyroscopes.
The satellite was lauched in 2004 and took measurements for 18 months until the helium ran out, it has then taken about 5 years to analyse the data properly, coming out with a figure of about 10.3 millionths of a degree per year for the frame dragging effect, which compares well with 10.8 millionths of a degree per year which relativity predicts. They also found a very good agreement for a second effect called geodetic precession.
These not only show how good Einsten's theories are, but allow astonomers to improve their models of heavy objects like neutron stars and black holes.
Is Earth's core cooling?
Chris - Well, it's a very good question. There are a number of answers to this. One of them is that the Earth is a fairly big planet, as planets go, and it had a bit of heat to start with. So the particles that coalesced to make the early Earth, when they came together and accreted, they actually had some energy so when they got together that energy was imparted into the Earth so it had some embodied energy to start with.
Then there's another effect to do with gravity. Because gravity works through an object's centre of mass, heavy things are pulled towards the middle and lighter things are therefore displaced above them. When the Earth first formed there would have been a mixture of the big stuff and the small stuff all mixed up. Over time, under the influence of gravity, the heavier things have settled towards the centre, and the lighter things towards the surface, and that would have generated some frictional effects and therefore you've turned some gravitational potential energy into heat.
Those two mechanisms are quite minor contributions to where the Earth's heat comes from, though they're not insignificant. By far the biggest player is what we call radiogenic heating. The Earth is effectively a giant nuclear reactor. There are particles in the Earth's core and throughout the mantle which are radioactive.
When things decay radioactively they produce heat. The vast majority of the Earth's energy is coming from the radioactive decay of these components, and they include things like thorium and also potassium.
Interestingly, there was always a big quandary: if potassium is a source of all this heat, why is there so little of it present in the mantle and outer surface of the Earth? It's a light element so we should find it in abundance at the surface. Well, it turns out that under very high pressures, potassium can form an interesting alloy with iron (a much heavier element). Of course, the iron is all in the core of the Earth, so what scientists think has happened is that the potassium has sunk with the iron down into the core of the Earth, and it's down there in the middle, decaying, producing a lot of the heat that we have warming up the Earth today.
Where is all that heat going and is the Earth cooling down? Not really, it's staying about the same temperature. Geological estimates are that the Earth loses heat at a rate of about 50 terawatts. That's about 50,000 1 gigawatt power stations worth of heat loss, i.e. if power stations pump out power at a rate of 1 gigawatt then you'd need about 50,000 of them - that's how fast the Earth is losing heat through the oceans, continental surfaces, volcanoes and so on, and that means that those processes inside the Earth that I've mentioned must be producing heat energy at a similar rate to balance things out because the Earth isn't cooling down that much.
Would switching a bulb on and off each second extend it's life?
Dave - There's at least two different failure modes for a light bulb filament. One of them is essentially evaporation. You've got a very thin coil of wire sitting there at 2,000 degrees centigrade, and although tungsten has a very high melting point, some of it is still going to be evaporating away all the time. There is a little bit of gas in there which slows it down, but eventually the filament is going to evaporate away to the point that there's a gap. That gap gets hotter and hotter and evaporates quicker and quicker until eventually it fails. So if you're turning the light bulb on and off, then that mode of failure is going to be a lot slower because it's only on half the time and most of that time it's either heating up or cooling down. So that mode of failure will probably take at least two or three times as long before it fails on average if you turn the light bulb on and off. But there's another mode of failure, which is just the wire breaking. If you're repeatedly heating something up and then cooling it down again then you produce all sorts of physical stresses and strains on it; as it heats up it expands and stretches, then as it cools down again it shrinks, so you'll get metal fatigue. I think the far bigger effect from turning the bulb on and off will be the wire breaking due to metal fatigue and repeated stretching, and it will break far sooner than it would do normally.
Chris - Can I ask you a spin-off question about this, Dave? If you are using AC current, where it goes 'plus', for the sake of argument, then down through zero into 'minus', and then back up into 'plus', it means that the bulb is effectively flicking on and off. So with 50 cycles per second, that's 50Hz AC, it means the bulb is flicking on and off 100 times a second. So does that knacker a bulb more than if you ran a steady current like DC through it?
Dave - It will have a slight effect, but the time it takes for the bulb filament to cool down is a lot more than 1/50th of a second, it's probably around 1/10th of a second. So the actual temperature of the bulb isn't fluctuating hugely due to the AC, so it's approximately the same as DC. Because the filament is effectively an electromagnet there will be some small extra forces acting with AC, though. As the electricity goes through the coil it's going to produce a magnetic field, and that will apply some slight forces and vibration on it, so probably AC is slightly worse for it but not by very much, because the heating up and cooling down time is so much longer than the AC time.

What is the difference between Homo floresiensis and African Pygmies?
Diana - First of all, yes we do think that Homo floresiensis is a different species - or at least most people do now. Pygmies in general are thought to be Homo sapiens, although the term pygmy is quite old now so it's better to use the local names like Mbenga and Mbuti and Baka and that kind of thing. But basically, floresiensis would live on the island of Flores about 100,000 years ago to about 12 -13,000 years ago. Some people have argued that it's a micro-cephalic human, which means that it had a really small head. The brain case was about Ã?,¼ of the size of modern humans today. While, of course, normal pigmy people have perfectly normal sized brains. The other thing is that pigmy is actually a term that was developed earlier this century to describe people who were slightly shorter than usual. I think African pigmies were 4 Ã?,½ feet, whereas floresiensis was around 3 Ã?,½ feet tall.
The African pygmies I have read about mostly lived in the forests, and as a result it's been found that they had slightly different ways of metabolising iodine, because it was a very iodine deficient environment. And that could actually have led to them being shorter. The other thing is that [African pygmies] are actually genetically quite divergent from the rest of us. It's thought that they diverged 60,000 years ago which is quite a long time ago.
How do web companies keep up with data storage demands?
Chris - We're all nodding our heads here. Yup, that's basically it!
Dave - The difficult bit is connecting them all together in such a way that if one of them dies it all carries on as if nothing happened. Chris - And I think it's worth re-iterating the words of Professor Andy Hopper from Cambridge University Computer Sciences who was in this studio a little while back. His stat was that the internet produces almost as much CO2 as the airline industry around the world. That's just data centres running to supply us with all the data that we are demanding.

31:48 - Planet Earth Online - The Energy Costs of Old Architecture
Planet Earth Online - The Energy Costs of Old Architecture
with Professor Bob Lowe, UK Energy Research Centre
Diana - If you live in an old or historical building, and let's face it here in Cambridge the chances of that are very high, then you're also probably aware that there can be downsides too. Sometimes all the features that make a building beautiful such a skylights, architectural touches and masonry, contribute to it being a cold and drafty dwelling. So how do you make buildings that are hundreds of years old energy efficient without tearing them down and starting all over again? Planet Earth podcast presenter, Richard Hollingham, went to London to find out...
Richard - I'm at the back of Paddington Station in West London, next to a fairly busy road, and with me is Bob Lowe from the UK Energy Research Centre. One of his areas of research is how to improve the energy efficiency of cities. Now Bob, you're a building physicist, so let's look at this building here. It's, what, a five story building. We reckon between us (neither of us are architects) about 200 years old. It's got a rather nice cream facade, large sach windows, surrounded by this ornate masonry. And there's a balcony running the length of the building. This is a nice building, you would not want to tear it down, but I guess it's not energy efficient.
 Bob - It probably isn't energy efficient. Things will have been done to it over the years, it's almost certainly got some double glazing - in fact, I can see the double glazing in there. It will have modern heating and ventilating systems inside. But basically the envelope is solid wall with no insulation, very high heat loss. So if you want to do something about the energy consumption of London as a whole, you actually have to deal with hundreds of thousands of buildings like this in order to correct that problem. And nationwide, there is something like 7 million solid wall buildings.
Bob - It probably isn't energy efficient. Things will have been done to it over the years, it's almost certainly got some double glazing - in fact, I can see the double glazing in there. It will have modern heating and ventilating systems inside. But basically the envelope is solid wall with no insulation, very high heat loss. So if you want to do something about the energy consumption of London as a whole, you actually have to deal with hundreds of thousands of buildings like this in order to correct that problem. And nationwide, there is something like 7 million solid wall buildings.
Richard - Just on the junction we're standing at, these buildings stretch all the way down the street into the distance, we are surrounded by them, so here's the question: how do you make this more energy efficient?
Bob - We did have a look at the back on our way round and one of the things about buildings of this age is that very often the back is much much more plain than the front. It actually turns out that you have more wall area at the back. Because the wall goes in and out with things that up in Yorkshire we call off-shots, I don't know what you call them down here - kitchen extensions and so on - built in when the buildings were first built. So you've probably got 50% more wall area and, in principle, you can insulate it.
Richard - The thing is, you wouldn't design a modern city, if you were trying to reduce its carbon footprint, like this. The junction here for example, with blocks of four roads going off in each direction, busy roads, and solid walls.
Bob - And yet there were some things that the Victorians got very very right indeed. These days, it's almost impossible to replicate this kind of urban environment under modern planning rules - with rules for roads, turning radiuses for traffic and so on, so this is very very difficult to replicate.
Richard - So what's good about this?
Bob - What we have here is very high density, it's then relatively straightforward to connect this to effective public transport systems, buses, the underground system, something that occurs in very few other cities in the UK. I can think of maybe 2 or 3 cities that have significant underground railway systems. So what we have here in transport term is extremely efficient. And you've got to remember that building energy consumption is only about 30% of total energy consumption in the UK, and transport is nearly as important and growing much faster.
Richard - So if you're looking to retrofit a city - to make it greener - you've got to look at all these things. You've got to look at the clad in the buildings, the transport system, where the energy comes from, the whole lot.
Bob - That's right. At the moment these buildings are almost certainly heated by gas supplied with electricity from power stations in the rest of the country. Well, in terms of the gas heating, it would be possible to heat the area here with hot water supplied from nearby power stations through pipes - something that's done in many towns and cities in places like Denmark and Sweden, parts of Germany and so on. That would require significant interventions across the city, it would require a level of planning which the UK has not been that good at over the last 20 or 30 years since I became involved in this kind of work. These are real problems that don't go away.
Why do we feel as though we're rocking after getting off a boat?
Chris - It's very common, it turns out. The records go back to the 1700s. Erasmus Darwin, who was Charles Darwin's grandfather, actually recorded one of the first, if not the first, description of this condition. And what Darwin wrote was:
"Those who have been upon the water in a boat or ship so long that they have acquired the necessary habits of motion of that unstable element, at their return on land, frequently think in their reveries or between sleeping and waking, that they observe the room they sit in, or some of its furniture to librate like the motion of the vessel. This I have experienced myself and have been told that after very long voyages it is sometime before these ideas entirely vanish. The same is observable to a lesser degree after having travelled some days in a stage coach, and particularly when we lie down in bed and compose ourselves to sleep."
He wrote that in 1796, it's reassuring to know that people are still having the same problem today. This actually is a medical condition. The fancy name for it is 'mal de debarquement', in other words 'illness of disembarkation', or feeling unwell when you get off the boat. For some bizarre reason, it appears that the incidence is highest in women in their 40s. It may just be that women in their 40s are more prone to going on cruises than people of other demographics, I don't know! But it's amongst them that you tend to get most of the medical reports. Luckily, it's generally a temporary thing. What scientists think is going on is that you have in your head a model of the world and how you are relating to it. In other words, if the world is moving, then you're modelling that movement and working out how to compensate for it either with movements of your head and eyes or your balance system so that you don't fall over. When you're on a ship, because of the constant movements, your brain has to 'de-tune', or damp down, that response a little bit. If it didn't, you would continuously be over-correcting for it, which might underlie why you get seasick in the first place, and why after a period of time at sea you stop feeling seasick. So, when you then come back onto land, the signals are being fed into this system which models how you're interacting with the world. When those signals are coming in now, you're not continuously in motion so the very thing that was expecting you to be in motion is no longer always seeing motion. As a result the model is predicting how you should respond to the movements around you incorrectly. So you experience these rather strange sensations as though the world is continuously moving. And it takes a little while to 'un-learn' this newly learned behaviour and to revise your model so you then don't keep jumping around all over the place.
Why does cooled water sometimes suddenly freeze?
Dave - It's a beautiful effect it's called super-saturation or super-cooling a liquid. What's going on is that OÃ?,°C is the temperature below which water is more stable as a solid than as a liquid. That doesn't mean you can't cool water below OÃ?,°C. That's because forming a very very small crystal takes quite a lot of energy and is quite unstable. So with water, it could be down to -3Ã?,°C, you can supercool water to almost -20 Ã?,°C, and if its below 0 every time a little tiny crystal starts to form it just gets knocked apart, so there are no small crystals there and nowhere the ice crystals can grow from, and the whole thing won't freeze. But if somewhere you get a crystal which is bigger than the certain critical size it's much more stable for that crystal to grow and grow so you get a few small crystals spreading out and the whole things freezes very quickly, because its below zero. You also get that effect with sodium acetate. There are hand warmers based on this principle. You heat them it melts the liquid inside, you cool them down again, you flick a little clicky thing and that releases some crystals which causes the whole thing to crystalise. This releases lots of heat which warms your hand nicely.

Why are native African people black?
Diana - Logically, it should be the case that if you have pale skin it's going to reflect more light and more heat and would be suited to warmer, sunnier places. But what is actually happening is people with darker skin have more melanin, more pigment, in their skin and this prevents the short wavelengths of light, the UV, from penetrating deep into the skin. It also means the skin can produce vitamin D in suitable levels.
So if you live in more northern climes where there is less sunlight, so for example in the UK, then it's much better to have pale skin which allows more sunlight to get into your skin and more vitamin D to be produced. Vitamin D is great for strengthening bones. It prevents you from getting diseases like rickets which would be very selective against a population if you were living in northern Europe.
Chris - We interviewed Nina Jablonksi, Professor of Anthropology from Penn State University in the US about this. She also made a good point about this, that UV radiation damages folic acid which you need for the development of the nervous system.
If you get folic acid depleted you get diseases like spina bifida; so, in continents like Africa where there is a lot of UV in the sunlight, if you don't protect yourself with lots of melanin you will deplete your folic acid, leading to an excess of neural tube defects like spina bifida and this would manifest in a cost to reproductive fitness in the population.
So, as there is so much sunlight, Africans can afford to have dark skin and still make enough vitamin D and not lose their folate.
But once you get up to the parts of the latitudes we live in (the UK), where it's miserable all the time, vitamin D becomes the real problem. You need to make enough vitamin D and so you have to have pale skin.
There's so little UV because we hardly ever see the Sun anyway that it doesn't become a problem from the folic acid depletion, neural tube defect perspective.

Balancing Brooms - finding the centre of mass
Balancing Brooms
Why put pennies on Big Ben's pendulum?
Dave - The period of the pendulum is entirely to do with the distance from where it is swinging to the centre of mass. The trick is where they put the pennies. They don't put them on the middle of the pendulum bob, they put them on the top. If you put a penny on the top of the pendulum bob, its adding some mass to the top of the pendulum bob so it effectively makes the pendulum slightly shorter, so it runs slightly quicker. If you put some weight on the bottom it effectively makes it slightly longer so it runs slightly slower.
Why do fingernails have horizontal marks on them?
Diana - Fingernails have two sets of ridges. You have vertical ones which go along the length of the nail and those ridges are just caused by variation in the cells that line your nail bed. You can also get horizontal ridges and I think these are mainly thought to be caused by trauma to the nail bed. You can also get illness events which cause all sorts of interesting things to happen to your nails. You get white ridges and the little white marks as well but I think that's generally attributed to trauma. Chris - I was thinking about why those marks might be white. I was thinking it might be similar to the reason that when you smash glass, or grind up glass and make sand again, or you make water freeze and make little ice crystals like snow - it's white, whereas the thing it started out as was translucent. If you look at the nail, it's a crystal structure of keratin, the protein, and if you traumatise it you probably get different fragments of bits of protein and water in there and other debris, all of which probably bend light all over the place and does the same thing as snow - so it reflects more light back, so you get a white patch. Diana - Yes, a lot of people seem to talk about zinc deficiencies or various other vitamin or mineral deficiencies that cause this but it seems to be something more trauma related.
Is it better to suntan lying flat?
Dave - I think it depends where you're living. If you're living on the equator, especially at midday, the Sun's going to be right above you at which point the only way you'll get the maximum amount of sun per square centimetre on your skin is to be at right angles to the Sun. If you're somewhere near the equator and lie flat that's probably going to be your best bet. If you're living somewhere a long way North, like Sweden or Finland, then the Sun is very low on the horizon and the best way to get yourself at right angles to the Sun is to stand upright, so I think often in Russia and Sweden you get people going out sunbathing standing up, next to a wall. I think the only advantage of not moving around is possibly that you fall half asleep and time passes more quickly!
Can we see atoms?
Chris - Yes, you can. In fact, it was the 30th anniversary recently of a fairly iconic image that was published by IBM. They actually wrote the letters IBM using atoms of xenon which they manipulated around on a surface using a scanning tunnelling electron microscope.
So you can see atoms, but you're not really seeing the atoms, what you're seeing is the electron field created by the electrons around the atom and how they produce a current when they interact with a very fine tip on an electron microscope.
One more recent study imaged atoms in a slightly different way. What this study did was to take a sheet of graphene, a single layer of carbon atoms, rather like a honeycomb which stack up make graphite. If you peel away single layers of graphite you get graphene. And what this particular group in America did was to sprinkle some molecules onto this graphene sheet and then run a scanning tunnelling electron microscope across the surface. And because the graphene is so regular in structure, it's easy to mathematically subtract the effect of the graphene being there from any other signals that you get. This enables them to actually see the structure and shapes of molecules.
If you wind your mind back to the days of chemistry lessons, when your teacher would draw a chain of, say, butane - four carbon atoms all linked together - and draw the kinky line of these atoms (that's the atoms being bent and kinked, not the atoms being kinky!) how do they actually know it looks like that? Well, if you look at these pictures they've published recently, they really do look like that - it's absolutely fantastic.
Why rinse with water to prevent cola frothing over?
Dave - This is an effect very related to the freezing water we were talking about earlier. Basically, there's lots of carbon dioxide gas dissolved in the cola, and as soon as you release the pressure, that's much more stable as a gas, rather than dissolved in the cola. But, in order to form a bubble you've got to create a whole lot of extra surface and that costs lots of energy. This means bubbles are only stable when they're above a certain size.
If you've got a very clean glass, there's nowhere where a bubble can form, so it takes a long time for the bubbles to form, and so it doesn't froth up. If you've got lots of dirt on the bottom, some of that can be hydrophobic, which makes it easier for the bubbles to form. You also might trap some gas, so you'll have a bubble there to start with. As soon as you've got lots of bubbles there, you'll get lots more bubbles, they'll grow very quickly, turn into a froth and make a horrible mess.
Chris - And I guess the spin off from that is that if you do clean the glass first, your drink will stay fizzier for longer if the bubbles are forming more slowly?
Dave - Totally. There will be more gas in there for longer, because it's escaping less quickly.
How does blood clot?
Chris - Very good question. I suppose the simplest way of thinking about this is that it's a bit like glue in a tube, in the sense that you've got various components inside the glue in the tube, and it's only when they get squeezed out of the tube and they meet the foreign environment of the air that they then get sticky, and stick things together.
In blood, there are several elements. They include little bits of cells called platelets, which are programmed to recognise holes in blood vessels and bind on to them. There are also proteins which are dissolved in the blood which are able to work like tiny pairs of scissors and cut other proteins to produce a cascade of changes which culminate in formation of a fibrin network. This looks like a fishing net inside a blood vessel which traps blood cells in it, and acts as a plug to block a hole in a blood vessel, for example.
So if we look at how it works: If you have a blood vessel and injure it, what happens first is that the platelets, which are circulating in the blood, recognise the fact that when the blood vessel is punctured there are now foreign surfaces exposed to the blood. The platelets bind on and then release various factors that trigger the blood vessel to constrict, so it gets narrower and reduces blood loss from the area. It also starts to recruit these other proteins dissolved in the blood, the coagulation factors, activating them sequentially. They cut each other, activating other coagulation factors, and culminating in the production of this cleavage of a protein called fibrinogen, to make this fibrin network.
All of this happens in a very short space of time - literally seconds for platelets blocking up the hole, to minutes for the formation of one of these fibrin networks. Once the gap in the vessel has been plugged, then the cells locally which line the vessel over-grow the area which has been breached, and they establish a new smooth lining to the blood vessel. The clot is then slowly metabolised away by other cells called macrophages. Dave - Does the same thing happen if you injure a blood vessel which is inside your body, and it's not exposed to the air and foreign environment?
Chris - Exactly right. The thing that the platelets and some of these other factors are recognising is collagen, the main building block of connective tissue. The proteins, including one called Von Willebrand factor, in the bloodstream are able to recognise the presence of collagen which is not normally ever seen inside a blood vessel or in a healthy organ, because they're normally kept separate from it. So whenever that interaction occurs, this tells the factors in the bloodstream that a vessel must have been breached, and therefore you activate the clotting system and it plugs up the hole wherever this occurs. Normally blood vessels keep themselves clear because the lining of the blood vessel, the endothelium, produces various factors which are anti-thrombotic, they antagonise or prevent blood from clotting. Obviously if you damage the blood vessel, you remove that anti-coagulative ability, so it shifts the blood into a pro-coagulation state, and then it starts to clot.

57:23 - How do we keep warm?
How do we keep warm?
We put this question to Professor Roger Thomas, from the University of Cambridge:
Roger - I'm Roger Thomas, a Professor of Physiology in the University of Cambridge and I do lecture to medical students on thermoregulation. What we should start with is saying that we're all producing heat all the time by the chemical processes in our bodies. For example, the heart, pumping blood around the body, uses energy derived from glucose, and the mechanical energy from glucose is produced with waste heat. So all the time the heart is working, it's producing excess heat. All the other chemical processes going on in the body, digesting food, thinking and so on, the amount varies, so after a heavy meal you produce a lot more heat as you digest it than normally.
I expect the question you really want to know about is producing heat when you need more than normal. There are two mechanisms there. One will be very familiar to most of you - shivering. This involves involuntary contractions of large muscles which generates heat wastefully, as it were, but in this case you need the heat to keep warm. The other mechanism is known as non-shivering thermogenesis, or heat produced by mechanisms other than shivering. This primarily involved brown fat. These are cells in patches of tissue all over the body, particularly near the heart, in the shoulder blades and so on, which are very important in newborn babies, who have a lot of trouble regulating their body temperature. In adults it's also found, although this has been controversial, and this works by simply burning glucose to produce heat rather than any other form of energy. It's a 100% heat production process, and this tissue is located near blood vessels, so it warms up the blood and warms the whole body.
Diana - Brown fat is great for burning glucose and producing heat. It has been hypothesised that people who live in houses without central heating tend to have higher proportions of brown fat.
But then, central heating is another form of thermo regulation - along with clothes and blankets.
And a paper published in Obesity Reviews in January actually showed evidence that living in warmer houses is contributing to obesity - and this could be related to reduced proportions of brown fat against white fat.










Comments
Add a comment