What is mirror life?
We’re taking you through the looking glass to explore ‘mirror life’: could we be about to flip biology on its head?
In this episode

00:49 - Louis Pasteur and the birth of chirality
Louis Pasteur and the birth of chirality
Phillip Broadwith, Chemistry World
In 1848, the revered French scientist Louis Pasteur made a remarkable discovery - some say in the bottom of a wine glass - that would help shape the future of chemistry and biology: molecules can be either left- or right-handed. It matters because this property, which is known as chirality, dictates the very structure of life itself.
But here’s the twist: life on Earth has always favoured one side or the other. Our proteins are all left handed; our DNA and the sugars we metabolise are all right handed. Why? No one knows. But now scientists are pushing the boundaries of biology by taking the first steps towards creating “mirror life” - organisms built entirely from reversed molecules: literally DNA that spirals left, and proteins that curl to the right.
The rationale for doing this is sound. It’s hoped it will advance medicine, biotechnology, and maybe even the search for alien life. But a number of world experts are worried, calling recently in a communication published in the journal Science, for a pause in research on mirror microbes. They argue that this technology could pose a grave risk to life on Earth. We’ll hear from one of those researchers later. But first, what exactly is chirality? And why does life depend on it? Phillip Broadwith, from Chemistry World, invited me out for a drink at the famous Eagle pub, in Cambridge…
Chris - Hello.
Phillip - Hi Chris, good to see you.
Chris - Yeah, likewise. You could have made it a bit warmer, Philip, but it's a nice day.
Phillip - It's lovely, but there is a reason why I've brought you here, Chris. This is the Eagle Pub in Cambridge, where James Watson and Francis Crick announced the structure of DNA, which is probably the most famous chiral molecule in the world.
And I shook your right hand with my right hand, because that's the only way it works. We could have done left to left, but that's a bit less conventional, unless you're a Cub Scout, but a right to left won't work. And we'll talk about that in a minute.
Chris - Let's get inside out the cold weather and you can tell me more about it.
Okay, right, let's put that down there. Well, we've got two nice cups of coffee. This is a pretty famous pub.
Phillip - Yeah, absolutely. So just around the corner are the Cavendish Laboratories, or the old Cavendish Laboratories, where Watson and Crick were working on the structure of DNA.
And legend has it that they came to the Eagle Pub and announced it.
Chris - You use that word chirality, and you shook my hand, right hand to right hand and said, there's only one way that can happen and chemistry is the same. You better explain what this word means.
Phillip - Well, the structure of DNA itself is a spiral, a helix, and that goes in one direction or the other. If you have a right-handed helix, it is different from a left-handed helix. It spirals the other way and they don't fit together.
They're different molecules. Lots of molecules have this property of handedness. The word chirality comes from the Greek cheir - which means hand.
And if you think of your two hands, they're mirror images of each other. They're not the same. You can't put them on top of each other and have your two thumbs over the top of each other if your hands are the same way up.
But if they're facing each other, they do. They're kind of mirror images.
Chris - And molecules are the same, are they? So if I take your hand analogy, and imagine my hand's a molecule and the fingers are different atoms stuck onto the core of the molecule, then you can have the atoms relative to each other in different places in space. They're the same molecule because they've got the same chemical formula, but it's how the atoms are arranged relative to each other, how they're stuck on effectively that gives it that one handedness or the other.
Phillip - Yeah, exactly.
If we think of the sugar that you might have put in your coffee, sugar is a handed molecule. It has left- and right-handed forms. And all of the sugars that we incorporate into our body have this same property of handedness.
Chris - How did this come to light in the first place?
Phillip - Lots of other molecules have this property as well. And what Louis Pasteur discovered was by looking at crystals of a molecule called tartaric acid or a salt of tartaric acid, like the little crystals that you might find in the bottom of a wine glass if we go on to some wine a bit later, Chris.
Chris - Wishful thinking. You better do a good job of this interview and I might think about it.
Phillip - So, he looked at these crystals of sodium ammonium tartrate and noticed by looking under the microscope that there were two different shapes of crystals that were mirror images of each other.
So, he then, very carefully, very painstakingly over hours and hours and hours took his crystals and separated them out into the left- and right-handed crystal forms and then dissolved them back up and noticed that one of those crystal forms rotated light to the left. The other one rotated light to the right.
Chris - So, literally then, you've got a situation where the handedness of the molecule makes a crystal that's asymmetric. One particular symmetry of the molecule makes a crystal that has that shape as well. And you end up with crystals that are mirror images of each other. And that's what gave the game away to Pasteur.
Phillip - Exactly right. And that's the same with our DNA with proteins. If you make them out of the same handedness of their building blocks, the individual sugars or the amino acids, then they get a handedness to their shape.
If you were to try and make them out of the opposite handedness, then you would get the mirror image overall macro shape as well.
Chris - Does this mean then that when life got started on Earth, if we go back four and a half billion years, that there were equal numbers of all of the different types of chemicals probably on the early Earth, but as life got going, it's enriched for one of them. It started using one of them or is something special going on?
Phillip - Well, we think that is right. There are meteorites that we found that have both handed forms of early molecules on them, amino acids and that kind of thing, or the precursors to life. But what we think happened is that there are a series of reactions where these molecules interact with each other and can replicate themselves.
And if you tip the balance ever so slightly towards one handedness, and then it self amplifies and makes more and more of its own handedness at the expense of the opposite, then over millions of years of evolution, as the molecules get more and more complicated, they're only incorporating this single-handedness of the molecules. And while the other molecules might exist, they're not enriched in nature in the same way.
Chris - Are they chemically identical though? So from a chemist's point of view, you're a chemist, if you did an experiment with one handedness or the other, would they have exactly the same reaction profile, etc? It's only in biological systems, they would have a different behaviour?
Phillip - Well, it depends what you're trying to interact with.
If you're trying to interact with something else that has chirality, then you will have some selectivity one way or another. So, if you think back to the Nobel Prize in Chemistry a couple of years ago for organocatalysis, asymmetric organocatalysis, with Dave McMillan and Ben List, that was all about using chiral chemistry. And it's extremely important chiral chemistry, a lot of drug molecules are chiral because we want them to interact with biological systems.
But to make those, we need to use chemical systems that are themselves chiral to impart chirality to the molecules.
Chris - What are the implications then of the fact that we have this biological world that's rooted in handedness?
Phillip - It controls how molecules interact with life.
It means that living systems tend to include one-handedness of molecules. If they include the opposite-handedness, then they might not interact with our systems in the same way. So one example is that there are bacteria that have small amounts of the opposite handedness of some amino acids on the outside of their bacterial cell walls, which makes them much, much more difficult for our immune systems to deal with because they don't see or they don't fit into the machinery that would normally chew up those molecules and help us get rid of those bacteria.
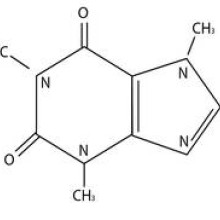
08:58 - Mirror life and backward biology
Mirror life and backward biology
Jonathan Jarry, McGill University
We now know what chirality is - but what about mirror life itself? Jonathan Jarry - a science communicator with McGill University's Office for Science and Society - has just written an excellent piece on exactly that, and I've been speaking with him…
Jonathan - When we talk about life as we know it, which includes bacteria, plant life, animals, us, it can be boiled down to DNA molecules, can be transcribed into RNA, and that RNA is translated into proteins and proteins are the effectors of the cell. They play all sorts of roles. But when we zoom in on the building blocks that make up these molecules, we realise that some of them have a handedness.
For example, DNA has a sugar backbone and that sugar molecule, if you manage to put it in front of a mirror, would have a mirror image, just like your left-hand is a mirror image of your right-hand. And when we look at what proteins are made of, which are amino acids, almost all of them are left-handed. And what's really interesting is that we don't see their mirror images in nature.
We don't see the left-handed version of this particular sugar, nor do we see the right-handed version of these amino acids, but we can create them in the lab.
Chris - So it's fair to say these molecules, the right-handed, left-handed forms, they exist chemically, they can exist, they do exist, but life doesn't use them. It has settled on a handedness, which presumably, given that it's in all the different realms of life on Earth, was a decision, in inverted commas, that life made very early on during evolution. And it's sort of fixed, that we've centred on using left-handed proteins, right-handed genetic molecules.
Jonathan - Essentially, yes, as far as we know, we have not seen the reverse of the mirror image of that in the world around us.
Chris - And do we see any examples of where this handedness of molecules is at play in biology, apart from the fact that life depends on building things with it that way? But do we actually see any other tangible examples of this at play, where it really matters whether you've got right-handed or left-handed molecules?
Jonathan - Well, basically, if we think of our immune system, for instance, a lot of the functions that it serves, defending us against these microorganisms that can cause disease, a lot of these functions are based around chirality, around handedness, which is that something in your immune system needs to bind something of this microorganism. There needs to be some kind of a handshake. And it works because these microorganisms, the kinds of molecules that we're used to, they're also made of. But if they weren't, that handshake might not be able to happen.
Chris - So, in the same way that Phillip shook my hand outside the Eagle Pub, and his right hand met my right hand, if I got infected with one of these bacteria, that was a mirror image bacterium, then it would be using the wrong handedness and it'd be like me trying to shake a right-hand with a left-hand.
Jonathan - Exactly. And so in theory, this would make this mirror bacterium invisible to you. And so there's an argument to be made that if it were to produce a toxin, for instance, that that toxin wouldn't be able to harm you, because it wouldn't be recognised by your body.
However, there was also this idea that this mirror bacterium, if it were to be growing inside of you, could be growing a bit like a cancer. And it might create a mass that might sort of push against some of your organs and lead to disease.
Chris - Is there a real possibility then that they would just exist alongside nature as we know it, and we would not be able to effectively see them, and we wouldn't be able to deal with them?
Jonathan - That's the thing. We don't really know what would happen because, in a way, the fact that their handedness is the opposite of what we're used to, it would create a bit of an invisibility field around them. They wouldn't be able to interact much with the world as we know it. They would be able to feed because there are nutrients that are what we call achiral. They don't have mirror images, or their mirror image is exactly the same as what they are, things like glycerol and butyric acid. Beyond that, what would happen to the environment? I mean, there is again, the potential that these mirror bacteria could invade the environment and without having any natural predator, sort of take over entire ecosystems and disrupt animal and plant life in certain ways. Those are things that scientists who are working in this field are getting increasingly concerned with.
Chris - How hard would it be though to do that? I don't mean as in for the organism to take over, I mean, for a scientist working in a lab to produce an entirely synthetic organism that is the mirror image, chemically speaking, of an equivalent that we already have?
Jonathan - There are two main ways of creating a bacterium like this, because some of these big molecules have been created, the mirror images of certain proteins have been created. And if you make enough of them, you can imagine at some point you could create a mirror microorganism like a mirror bacterium. And again, there are two ways of going about this. You could create all of these molecules in the lab, put them inside of a membrane, and hope that - like with Frankenstein's creature - it sort of comes alive. Or you could take a regular bacterium and reprogramme its DNA so that it would start making mirror molecules. So that eventually, like the Ship of Theseus, all of its parts end up being replaced by mirror molecules, and now it has become a mirror bacterium. And there was a report that was released recently by scientists who are concerned about the possibility of this. And their best guess is that, within 20 years, this could be possible.
Chris - If you could make a protein that had some amino acids that twisted the wrong way, so they were invisible or couldn't be broken down by normal digestive processes in the body, could you not make much better, more potent drugs? Because those proteins could do things, but they would be immune to the body's normal mechanisms of elimination.
Jonathan - Precisely. So, many of the older drugs that we have, they are not proteins, but many modern medications are these long chains of amino acids. We can think of Ozempic / Wegovy - that's a chain of amino acids. Many targeted cancer drugs are also chains of amino acids. There's a whole slew of biologics that help with autoimmune diseases like rheumatoid arthritis, psoriasis. These are also chains of amino acids. And as you pointed out, scientists are now retooling proteins and some of these drugs to replace a left-handed amino acid with a right-handed one, because when you do that, the enzymes in your body can't recognise the molecule quite as well. And so the idea would be that you would need a lower dose and fewer injections because that drug would stay in your body for a longer period of time before being degraded. And you could thus benefit from it for a longer time in between doses.
Chris - Where does this leave us then, Jonathan? Because on the one hand, you can see that this sounds like it could get dodgy and dicey quite quickly and there could be an unmitigated disaster awaiting mankind if we do it. On the other hand, as you've just said, there could be some enormous potential benefits. So is there a middle ground or is it just too dangerous and we don't play in this space?
Jonathan - Well, there's a difference between creating mirror molecules and creating a full-on mirror microorganism. It's my interpretation that that is where the line was drawn by the authors of that article that was penned last Christmas called “Confronting Risks of Mirror Life.” What I really hope happens following the publication of this piece is that there is even more discussion happening within these fields of study to decide what are the dangers, what do we know and where should we draw that line?

17:07 - The risks of mirror life research
The risks of mirror life research
George Church, Harvard University
As Jonathan just mentioned, dozens of scientists - including prominent figures like Harvard geneticist George Church, whom we’ll hear from next - recently published an article in Science called "Confronting risks of mirror life." Whilst the immense potential of chiral drugs cannot be denied, they expressed concerns about the potential creation of mirror-image organisms - particularly mirror bacteria. And as we heard from Jonathan, the means of creating self-replicating mirror life is fairly readily available already to any biochemist seeking to do so. And while opinions vary as to how long it might take to make such an organism - some say 5 years and others 30 - these aren’t all that far apart in the grand scheme of things. So what are the biological risks that need considering before going further with creating chiral life? And how can this be enforced? George Church…
George - There are risks to almost anything biological because it replicates. You can have invasive plants and animals, for example, and they're much riskier when they replicate than if you'd bring in a sterilised plant or animal. And hence, any responsible synthetic biologist who's doing this kind of work is going to do what's called biocontainment, and sometimes physical as well as biological containment, to make sure that that organism cannot replicate outside of the lab and whatever laboratory facility they're using is commensurate with its risks.
So, for example, people work on very serious pathogens in what's called a BSL-4 facility, which has very high physical risk mitigation, people wearing moonsuit type things. But in addition, there's biocontainment that can work quite effectively. So, I think that would be the mitigation.
And then the worst case is you get somebody who undoes that biocontainment intentionally so that they can develop it further and make it something that would be an existential risk for everybody. At least the theory that is worth considering, I wouldn't say it's proven by any means, is that most forms of life are unprepared for dealing with mirror forms.
Chris - Indeed, because most of nature's recycling system is a biological one, isn't it? You've got microorganisms, including fungi and bacteria that break stuff down. Chemistry and ultraviolet light help, of course, but one could foresee then if we made stuff that was the wrong-handedness that the biological recycling system couldn't see, would we end up with the biological equivalent of plastic pollution, where we're making things that will just go out into the environment and have a really, really long lifetime in the environment? And they could have all kinds of biological effects because of accumulation.
George - Well, that's one way of thinking about one of the risks. But I think a much more serious one is that these could invade every tissue in plants and animals and effectively act as pathogens for which there's no immune system. So, long before you start accumulating tons of mirror bacterial waste, you're going to have them eating the food that is floating around in your blood and in the plant fluids, making them less healthy as a result and eventually killing them.
Chris - In the past, when we've had dangerous technologies, there's been some safeguard because in some cases they were so hard to work on - or so hard to do - you needed to be a superpower to do them. Like the atomic bomb, for example. Are we not in an era now where you can do quite a lot in your garden shed if you buy relatively simple, relatively cheap bits of kit? So is it going to be quite hard to regulate this? Once this genie is out of the bottle, people could start just doing whatever they wanted.
George - Yes and no. For example, with ammonium nitrate you can make explosives in your garden shed and similarly you can make poisonous gases like they used in World War I. But I put a white paper out in 2004 that suggested a specific way that one could regulate and, in particular, do surveillance. Up until then, it was kind of popular to say, we're going to have a moratorium where everybody says they won't do it. Well, that doesn't help you with the people that are doing it surreptitiously. We need surveillance and there are specific choke points where you can see that they can't practice a lot of most synthetic biology without being able to synthesise DNA. And we've got almost complete industry agreement on checking all the DNA orders, whether it's on a desktop machine or whether it's over the internet. And I think that would help.
Also, monitoring all of the mirror image precursor molecules, whether it's for DNA synthesis in machines or whether it's just using it in other experiments. All of that could be regulated. Now, it's not perfect, but it's a step that we can take.
Also, just developing more widespread diagnostics would see these things the instant they arise. Having secure facilities which would exclude them would be another thing. There are things that can be done in advance and probably should be done if we really take this seriously.
Chris - And where do you stand on it then, off the back of all that? Are you in favour of we just don't do this with mirror life at the moment? Or, do you think it's one of those things that is worth pursuing, if done safely, in the right hands, notwithstanding the risks, because there might be a lot of benefit from doing it?
George - It depends on what the ‘it’ is. So I think mirror image molecules are extremely valuable and have been for decades. Like the difference between the thalidomide that caused a lot of damage to infants and other thalidomides that are useful is based on its chirality. So, chirality is important.
Making non-replicating mirror DNA RNA protein is fine. I think we should be very cautious about replicating molecules, but we certainly should have a programme in trying to detect them. So if they do exist on Earth, we should understand how they're being kept in check. If they occur on a planet, we should find that out before we send anybody there and so forth. So, I think there's three ‘it’s’ here. One is mirror molecules that don't replicate. There's mirror life, which we don't want to create. And then there's detecting mirror life. And I think the first and the last are very promising and important.
23:54 - Could another planet host mirror life?
Could another planet host mirror life?
Lewis Dartnell, University of Westminster
Finally, if mirror life exists, it would show that life can develop with a different molecular handedness than what we see on Earth. Even if we never find it, studying the possibility helps us understand how life works. So, could it exist on another planet? And could it be problematic? Here’s Lewis Dartnell, an astrobiologist at the University of Westminster…
Lewis - When we're searching for life on other planets, we're expecting to find organic life, certainly on places like Mars or Europa. Carbon chemistry based life. And let's suppose we do find life on the surface of Mars or maybe molecular fossils, one thing that would be a really good sign that that life is different from terrestrial life would be if those biomolecules on Mars had the opposite handedness. So rather than life on Earth, which uses on the whole left-handed amino acids and right-handed sugars, if we found the opposite situation on Mars, that would be a very, very clear signal that that Martian life is genuinely alien, that it came from a completely separate origin, and we're not finding contamination from Earth.
Chris - So aside from its interest and its academic and possibly industrial applications, the mere fact that you might find mirror image life would be a really, really good endorsement of its veracity, if you like.
Lewis - Yeah, absolutely. And certainly with astrobiology, that chirality would be a very, very useful indicator that the biosignatures, the signs of life we found are from life which is very different from us, very biochemically distinct and we're not just finding contamination from the lab tech when we built the rover or the Mars probe. Or possibly that there seems to be the distinct possibility that life could have been transferred between the inner planets during the earliest chapter of solar system history.
So during the late heavy bombardment, when all the rubble left over from building the planets is still flying around through space and chipping off bits of Mars and Earth as meteorites, it's almost like the inner planets were sneezing in each other's faces constantly. So there seems a distinct possibility that life could have been transferred from Earth to Mars, or maybe vice versa, from Mars to Earth in the very early Solar System.
Chris - The rovers that we've got trundling around on Mars, some of them are quite advanced analytical laboratories in their own right, aren't they? But can they detect chirality? Are they equipped to do that? And have we found anything in that respect yet?
Lewis - We're essentially sending miniaturised laboratories to Mars with wheels on the bottom, so they're mobile and can find the most interesting spots, and solar panels on the back or other power sources to drive them around. And this chirality, that signature, is exactly the sort of thing that we're trying to detect on Mars. And although we have found organics on Mars, we've not detected yet any unambiguously biologically produced biomolecules.
So that would be the next step, the hopeful next step for astrobiology.
Chris - When we make one particular handedness of a molecule, there is this process of what they call racemisation or spontaneous isomerisation, where in fact the things can rearrange themselves to end up the other handedness. And that happens with time, just by chance, doesn't it?
So is there a possibility, if life went extinct on another body in the Solar System a long time ago and it was one handed, could it not have mixed everything up since then? And so we end up seeing a balance and we just assume, well, there was no life because there was no chirality, no bias in handedness.
Lewis - Let's assume we don't find life which is extant on Mars. So we find molecular fossils, the breakdown products of what once were cells, or at least we believe once were cells. And over time with just thermal degradation, just chemical breakdown, and the action of things like cosmic rays, radiation from outer space bombarding the Martian surface (which is my own field of expertise) these would all act to break down the large, complex, very obviously biological molecules, things like DNA or proteins, break them down to simpler molecules, the monomers that they're built from, which we'd expect to find anyway. And you're right about that racemisation process as well. If you start with a distinct bias in either direction in the anatomers, over long periods of time, that starts getting pulled back towards an equilibrium to equal ratios of both.
So it's almost like time is erasing, is rubbing out those bias signatures and making it harder and harder to have unambiguous evidence that there was once life on Mars.
Chris - Are we being adequately cautious? And have we always been adequately cautious when we've sent things farther afield to land on far away worlds to make sure that we don't pollute them? Because it seems to me that there's a possibility we could quite easily upset the apple cart.
Lewis - We try very hard with exploration of other planets to sterilise our probes as far as is reasonably possible. And this falls under a set of international laws called planetary protection.
We don't want to contaminate Mars with the very thing we're trying to find there. But the next step of exploration would be a mission called Mars Sample Return. So rather than sending a miniaturised laboratory to Mars to do all of our experiments in situ on the surface, we'll send a mission, send a probe, to collect some promising samples, maybe dug up from the ground, maybe rock samples, and then launch those back to Earth. So we can then use all the laboratories around the world to scrutinise these samples of Mars and try to find bias signatures. And in that case, we'd be facing the opposite problem. We want to not accidentally contaminate Earth with any life that might have come from Mars. So people are already designing and building containment facilities to make sure these pristine samples of Mars are kept completely isolated from the outside world when they return to Earth.
Chris - Wouldn't it be safer to do that sort of thing in space? We have some kind of orbital laboratory like the ISS and do the analysis off planet.
Lewis - This is quite a common trope of science fiction. It's just incredibly expensive to launch an entire capable laboratory into low Earth orbit. And I suspect it might be the worst of both worlds.
If you're having to try to miniaturise your laboratory equipment, your instrumentation, to make it flight capable, to make it capable to fly to Mars, you're not really gaining much by having to use similarly miniaturised equipment just in low Earth orbit, rather than using all the very, very capable instrumentation laboratories we have here down on Earth.
Chris - Those samples you're referring to, they're already being collected on Mars, aren't they? Perseverance, the rover that's there, is picking them up. But what are the prospects of actually getting them back? Because that would really clinch it, wouldn't it, if we can get those samples and give them proper rigorous analysis? Is it going to be in the near term or are we looking at decades to get those samples?
Lewis - With space exploration, it always comes down to getting the budgets in place, getting the missions designed, and then actually built and launched. And although Mars Sample Return has been on the horizon for a while yet, it's not yet been completely fully funded and given the final green light to go ahead. And who knows what's going to happen in the current American administration and government with funding for NASA and for science missions like this.





Comments
Add a comment