Gene Therapy and Stem Cell Therapy
This week's podcast is live from the British Society for Gene Therapy (BSGT) conference in Brighton, UK. Some of the world's top gene and stem cell therapists explain how we can manipulate genes to treat a variety of disorders, from Cystic Fibrosis and Haemophilia to Cancer and Blindess. We hear what life is like as a haemophiliac and answer your questions, including whether gene therapy can alter all the cells in our bodies and how scientists account for the ethics and side effects of this research. Plus, is a human moustache like a cat's whiskers? Find out in Question of the Week!
In this episode

- Could we use gene therapy to enhance cognition?
Could we use gene therapy to enhance cognition?
Simon - I think that would be a very exciting idea and I think there's been a film about it recently, Planet of the Apes. But really, even in the simplest of genetic diseases, what we're learning is that we don't understand everything about the pathology and how the genetic mutation makes the disease. And so, as I mentioned about glucocerebrosidase deficiency [Gaucher's disease] we find actually that it's a very complex disease even though it's just one gene missing. And so therefore, there are many, many different genes that are contributing to different aspects of thought and I don't think we'd even know where to start in terms of enhancement of thinking.
06:00 - Living With Haemophilia
Living With Haemophilia
with Adam Jones, University of Sunderland
Chris - One of the topics covered at the BSGT/ESGCT conference is haemophilia. Meera Senthilingam met an academic with a personal interest in the topic...
Adam - My name is Adam Jones. My profession is I'm a lecturer in physiology for Sunderland University and today, I'm here at the BSGT/ESGCT representing patient views as I have severe haemophilia B. With haemophilia, you have different types of haemophilia and you have different severities. The main ones that people most know about are haemophilia A and haemophilia B. Essentially, it's a disorder of blood clotting. In any haemophilia, one of the proteins in the blood that goes towards the clotting cascade isn't manufactured by the liver. And so, in order to correct that, there's two options - either a liver transplant which isn't a very good option or factor replacement therapy.
 Meera - This is actually what you use, clotting factor 9.
Meera - This is actually what you use, clotting factor 9.
Adam - Clotting factor 9 - that's specific for haemophilia B because it's the 9th one that's missing, Christmas factor. In haemophilia A, it's clotting factor 8.
Meera - And having haemophilia, what exactly happens to you? What are your symptoms?
Adam - I bleed, basically, for longer than most other people. There's a myth that says that haemophiliacs don't stop bleeding. We do stop bleeding eventually, when the blood runs dry (laughter) nah! We do stop bleeding eventually, but it's a prolonged bleeding and it can cause all sorts of worries, all sorts of damage.
Meera - How does this affect your lifestyle? So what do you perhaps have to do differently or what you have to think about, knowing this could happen?
Adam - I can't play contact sports. I have arthritis in my ankles and my elbow because of internal bleeding. It does restrict you in your chosen profession. I can't do anything physical or labouring which is why I went down the academic route. Some other haemophiliacs aren't as lucky as me. There are obvious limitations everywhere. Growing up as a child, I wanted to be in the Royal Navy. I even went to get my interview at the Royal Naval career office, passed all the selection tests, got as far as the medical, and when I told the doctor who was performing the medical that I had severe haemophilia B, he stopped it right there.
Chris - That was Adam Jones, giving a patients perspective on living with haemophilia.
08:18 - Targeted Gene Therapy for Haemophilia
Targeted Gene Therapy for Haemophilia
with Kathy High, Children's Hospital of Philadelphia
We've heard from
Adam Jones, Lecturer at the University of Sunderland about living with haemophilia. Kathy High, from the Children's Hospital of Philadelphia, offers a gene therapy perspective on treating the disease...
Kathy - Well, a long term goal of research in the field has been simply to get long term expression of a donated factor 9 gene in a human subject. So the disease was first corrected in mice about 15 years ago and the effort has been to move successfully from mice to haemophilic dogs, and on into people with haemophilia. The work that we've been doing that I talked about today though uses a different strategy, not just giving a new normal copy of a factor 9 gene to somebody with haemophilia or to an organism with haemophilia, but rather to go in and seek to actually correct the mutant sequence in an animal with severe haemophilia and restore a normal sequence so that that corrected gene could now be under the control of all the normal regulatory signals about when to go up and when to go down and so forth. And so, that's the work that I was talking about at the meeting today. It was accomplished using a synthetic molecule called a zinc finger nuclease that was delivered along with a donor sequence. The zinc finger nuclease cleaves the DNA at the site that you want to correct and then the cell's own mechanisms use the normal donor to make a repair to the cleavage sight that now installs the normal sequence instead of the mutant sequence.
Chris - And in that way, the protein, the haemophilia protein missing before is now being made correctly, and so as a result, the person should have a restoration of the blood level so their clotting should go back to normal.
Kathy - Correct and so, what we've done is show that that can actually be accomplished in a haemophilic mouse.
 Chris - But mouse don't get haemophilia, so this is a mouse you've made?
Chris - But mouse don't get haemophilia, so this is a mouse you've made?
Kathy - Right, this is a mouse. Well actually, mice probably do get haemophilia but they don't survive in the wild with haemophilia. So yes, this was a mouse that we made in the laboratory and installed the haemophilic mutation and then showed that we can correct it using this approach.
Chris - So if it works in the mouse, will it work in a person?
Kathy - Well most therapeutics in haemophilia have actually been tested first in a naturally occurring model of haemophilia - the haemophilia dog - and colonies of haemophilic dogs are maintained at a few universities worldwide and so, our next step will actually be to attempt to carry out the same correction in the haemophilia B dog model.
Chris - So talk us through the method that you'll use in the dogs then? What will you do?
Kathy - Well, we do essentially something very similar to what we've done in the mice. We have to design a different set of zinc finger nucleases because the dog factor 9 sequence is not identical to the sequence in the mouse that we corrected. But it will be located in approximately the same place so we'll give the dog an intravenous injection with an AAV [Adeno-Associated Virus] vector that expresses the zinc finger nuclease. And that will induce this double strand breaks in the dog liver and then at the same time, we will have given this donor with the corrected dog sequence and the cellular mechanisms in the dog liver cells will be triggered and will repair the site where the cleavage is and it should allow expression of normal canine factor 9 in the animal.
Chris - Why are you using that particular virus, this AAV9. What's special about it?
Kathy - So that's a good question and I have to admit that we chose it out of convenience and efficiency, but were you to consider expression in humans or to consider moving this forward into human subjects, it's going to be very important, I think, to identify a vector that will express only short term. Otherwise, I think you may have safety issues that arise from having the zinc finger nucleases expressed continuously.
Chris - Sure and the fact that it just goes into the liver, or does it go elsewhere? Do you know that for a fact?
Kathy - Well actually, we know that it only expresses in the liver because it has a liver-specific promoter, so it may go to other places. In fact it does at little levels go to other places, but the promoter does not allow it to express the zinc finger nuclease.
Chris - So I think this is the system that controls what genes get turned on and they'll only turn on in the liver.
Kathy - Correct, they'll only turn on in the liver.
Chris - And so, they make that change in the liver and then hopefully, life longer thereafter, you should preserve the expression of these new proteins.
Kathy - Well actually, one of the great features of this strategy is that it's a correction in the genome itself and therefore, it will be passed to every daughter cell. And so, even if the cell gets old and wears out, as long as there are corrections in the stem cells of the liver that give rise to the new cells then you'll propagate the change and we did actually show in the mouse model that we could remove 2/3 of the liver and then as the liver regenerates, you maintain the correction because the residual cells have the correction installed and as they divide and give to new cells, the correction is maintained.
Chris - So what proportion of the clotting factors in the blood are now the correct one when you do this?
Kathy - We were only able to correct something like 3 to 7% of the target alleles so that won't give you 100% level of factor 9. It'll only give you a modest level in the range of 3 to 7%, but haemophilia is one of those wonderful diseases where restoring even a very modest level of normal clotting factor, in the range of 5%, converts the disease from a severe one to a mild one.
Chris - And if this works the way it does in all these other mammals, it looks encouraging then that you should be able to translate this to the human.
Kathy - There will be additional issues that I think need to be addressed very carefully as this moves into human subjects for testing and we talked about those in the conference today. We need to make sure in the human genome that these particular zinc finger nucleases don't cleave other target sites - those are called off-target effects. So we've analysed those pretty thoroughly in the mouse, but the real tissue of interest of course is human cells, so we'll need to do further analysis in human cells. So those are the kinds of issues that will need to be addressed before this kind of strategy moves forward for in vivo gene correction, but this type of strategy is already in place for cells that can be manipulated in the laboratory. So, it's in place for example in T-cells in a trial that is underway for HIV. Of course, moving gene transfer from ex vivo to in vivo involves another series of considerations that will have to be addressed.
Chris - One other question that people often raise is, what about the question of spread of the virus outside of the person you're trying to treat? Is that a risk here or is it constrained and confined just to the person you are administering it to?
Kathy - Well you know, that's an interesting question and when clinical gene therapy studies first started, people were very concerned about the risk of what we call horizontal transmission, will the household contacts be at risk for being infected with the virus. So in the initial studies of AAV gene transfer, that was very extensively looked at. People were kept in the hospital for 24 hours and we had to collect all their body fluids and try to make sure that the body fluids were not infectious, that they couldn't transmit that to their household contacts. And so, fortunately that question has been resolved and that doesn't seem to be a big risk.
17:25 - A Virus to Combat Cancer
A Virus to Combat Cancer
with Iain McNeish, University College London
Ben - This week, we're at the British Society for Gene Therapy's annual conference in Brighton. One of the sessions looked at how gene therapy can be used to combat cancer; and the way researchers are trying to do it is by using viruses. Meera Senthilingam went to speak to Queen Mary University of London scientist Iain McNeish...
Iain - We talked about ovarian cancer and gene therapy using Adenoviruses in particular and our results show that ovarian cancers that have mutations in the genes BRCA1 and BRCA2 appear to be very sensitive to adenoviral gene therapy. Now, these two genes are mutated in around 40 to 50% of ovarian cancers and it means that women whose cancers have these mutations should be very sensitive to gene therapy, and obviously, these are the people we should be choosing to take part in our clinical trials. The other thing that we found is that ovarian cancers that are resistant to the chemotherapy drug Paclitaxel seem to be very sensitive to adenovirus and that raises two really interesting things: Firstly, resistance to chemotherapy - we found a whole new pathway whereby tumours can be resistant to chemotherapy that's never been described before. But also secondly, that if we treat people with Taxol first, then we should be treating them with adenoviruses afterwards. So, what I hope our result shows is that we found two obvious groups of patients who should be receiving gene therapy in clinical trials in the future, but also, it's revealing new mechanisms of how the virus works, how chemotherapy might work, and how cancers can become resistant to it.
Meera - And what would the adenoviruses contain to actually target these cancers?
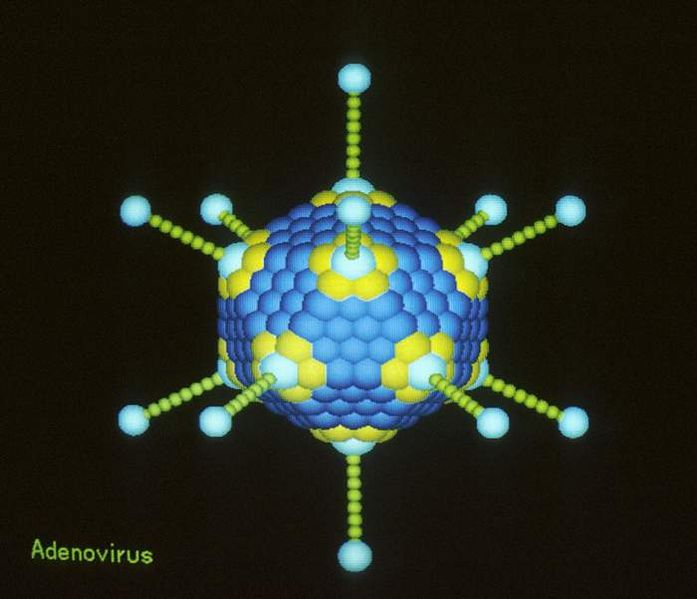 Iain - In fact, the adenoviruses contain nothing. So what we've done is we've taken a small part of the adenoviral genome out so that they now only replicate selectively within cancer cells. So, it makes them very, very simple to make because you're not putting new genes in. You're literally taking an ordinary virus, removing a small part of its DNA, and that makes it selective to kill cancer cells.
Iain - In fact, the adenoviruses contain nothing. So what we've done is we've taken a small part of the adenoviral genome out so that they now only replicate selectively within cancer cells. So, it makes them very, very simple to make because you're not putting new genes in. You're literally taking an ordinary virus, removing a small part of its DNA, and that makes it selective to kill cancer cells.
Meera - Why does it only replicate in cancer cells?
Iain - So the small part of the genome that we've removed interacts with a protein called retinoblastoma. We know that in cancer cells, retinoblastoma and the 10 other proteins that link with it are almost universally abnormal. So by taking these small little bits of DNA out, the virus can only replicate in cells that have an abnormal retinoblastoma pathway which is basically all cancer cells.
Meera - So this gets around any safety concerns about the virus spreading elsewhere?
Iain - In theory. Clearly, we haven't put our virus into trials yet. However, a very similar virus was put into trial in the states last year in women with ovarian cancer and it's still undergoing trials in people with brain tumours, and there've been no safety problems there are at all. Quite excitingly, showing that you can get anti-tumour efficacy by injecting similar viruses into people with ovarian cancer and brain tumours.
Meera - What actually causes the killing of these cancer cells, just purely the replication of the virus?
Iain - Ah, The billion dollar question, I think if we knew that! So, we think it's probably multi-factorial, but viruses replicating within cells do kill them. But also, that killing triggers an immune response. So we think it's probably at least two things: The direct killing of the cell by the virus but also, that sounding an alarm bell to the body's immune system saying, "Come and kill me!" So, trials that we did a couple of years ago with a Herpes virus, we were able to demonstrate not only where we're getting virus killing, but we were also getting immune cells coming into the tumour nodules, and we think that those two things combine to cause the overall effect.
Meera - In terms of what has been done so far, are there any figures as to just how effective this is in treating cancers?
Iain - Most adenoviruses are only at very early phase I, phase II [clinical trails]. The most famous adenovirus is the Onyx-015 virus which is now licensed in China - it's called H101. It's licensed for the treatment of head and neck cancer. So if you get head and neck cancer in China, you can get routinely treated with adenoviral gene therapy. The viruses that we work on, the second and third generation viruses are still in phase 1 and phase II trials. So, it would require another 3, 4, 5 years at least before we're into big scale trials before we can say for certain whether they really will help people. Conceivably, within the next 5 to 10 years, gene therapy with adenoviruses could be routine care in this country.
Meera - But they are as to the lab and so they have been trialled in humans.
Iain - Yes, they have. So, there are lots of viruses in clinical trials, not just adenoviruses - reovirus, and vaccinia viruses. So, this is not just lab stuff. These are viruses that were being used to treat people with cancer right now. In clinical trials, yes so they're not yet licensed. They're still experimental but they're progressing from the lab towards the clinic and the patient.
Meera - And what's the real end goal? I guess, if the trials progress and results remain positive, is a hope that it would be the treatment of choice above current treatments or would it be say, that you would identify people resistant to Taxol and so on, and then result to this treatment?
Iain - Well, the end goal is that cancer gene therapy becomes a standard of care. It'll be one of the options available to us, along with surgery, and chemotherapy, and radiotherapy. And the way we're going to treat cancers in the future, the buzz expression is "personalised cancer medicine" is that we're going to identify specific mutations and abnormalities in cancer and say, "Okay, you need to have this treatment and this treatment because your cancer has these mutations." And if we found a virus that specifically targets cancer of abnormal RB pathway and defective homologous recombination in BRCA mutations then you say, "Okay, here is a treatment option for you." So, it will be mixed in with other types of chemotherapy and radiotherapy, but it'll just be another string to our bow to try and treat cancer.
23:13 - Foetal Gene Therapy
Foetal Gene Therapy
with Simon Waddington, University College London
Chris - With genetic disorders, one concern is that the damage caused by a particular disease could peak early on in development, even while a baby is still in the womb. With such conditions, gene therapy before birth could improve a child's life expectancy. This is a field that Simon Waddington from University College London is working on. Hello, Simon.
Simon - Hello, there.
 Chris - What's your research actually looking at? What are you doing?
Chris - What's your research actually looking at? What are you doing?
Simon - One of the things that we look into is the concept of delivering the genes in-utero, in other words before birth. There are many diseases, many genetic diseases, where the onset is very early and can be lethal. So in other words, the baby actually is born with a genetic disease and that genetic disease has already damaged the baby's tissues to the extent that you can't reverse the illness. And so, what we're looking at is the idea of actually treating or preventing the disease from ever occurring by transplanting of the genes while the baby is in the mother's tummy.
Chris - How would you make the diagnosis? How would you know the baby has or is destined to get the condition?
Simon - That is a very interesting question. I think the first thing to say about this is that quite often, a family has a baby, finds out the baby has a genetic disease, and the child may then die. And then, we know of course that the parents are carriers of the disease and so, that actually is one way to then start to screen for the disease. Another thing to say is that for many diseases that are early and lethal, genetic testing is not performed because there is no treatment and so, there is not the same sort of driver to actually test the whole population.
However, the improvements that we see in genetic testing now - the speed of the technology and the accessibility of the technology - means that we can screen for a lot more genetic diseases a lot less expensively. The other thing is that over the past couple of years, there have been some very exciting developments where you can actually isolate cells that are circulating in the mother's circulation but they actually come from the foetus. And so, you can isolate those cells and then you can actually test the genetics of the foetus without actually interfering, sticking a needle into the foetus, at all.
Chris - So you could do genetic diagnosis from nothing more than just a maternal blood sample.
Simon - That's right.
Chris - This will give you some warning especially if you know it might be an at risk population.
Simon - Yes, that's right.
Chris - But is doing gene therapy on an unborn baby just the same as doing gene therapy on a person who's actually say, an adult? Is there a different constraint entirely in trying to do that? Will the techniques that we're investigating in petri-dishes and in adults actually apply in utero?
Simon - In one sense, at least in our research, it's easier to deliver the genes to a foetus than it is to an adult. With an adult, the immune system has already seen these viruses and you've actually developed an anti-virus immunity. One has to bear in mind that many gene therapy vectors are based upon an archetypal virus. So that's one problem that we avoid by going in early into the foetus. Another point is actually that you can put more copies, more genes, into the foetus and therefore, you might actually be able to get a wider distribution in the foetus too.
But I think that one of the things that we have to consider is that you're actually dealing with two patients of course. You've got to consider the health of the mother as well as the foetus and this is something that we've obviously been considering in our research for the past 10 years.
Chris - How do you do your research? It's already a very emotionally charged area - looking at pregnancy and children that haven't been born yet that might have a problem. Is there an animal model you can induce which will give you a faithful representation of what might happen in a human?
Simon - We work primarily with mouse models and so, actually we're able to deliver the genes into the foetal mouse by the same routes as one would be able to do a blood sample in a foetal human. Obviously the mouse model is a long way away from the human in terms of physiology but the principles are the same, and I think we're seeing this through the various gene therapy trials that are being performed anyway. Obviously, you have to test the safety of these in lower animal models before you scale it up to human beings.
Chris - What techniques are you investigating that you're trying to use? What gene transfer techniques are you investigating that you're trying to use?
Simon - What we're looking at, for example, is that we've been interested in neurodegenerative diseases. One particular interest of ours is Gaucher's disease which is a deficiency in the enzyme glucocerebrosidase. Humans with the severe form of this disease, called neuronopathic Gaucher disease, will only expect to live for 2 years after birth.
 We have a mouse model of this where the mice only lived for 14 days after birth. We're using a vector called AAV9 which is Adeno-Associated Virus 9. People found a couple of years ago that when you injected this in the circulation, you could cross the blood-brain barrier and could actually deliver the genes to the central and peripheral nervous system. This is the main problem with neuronopathic Gaucher disease. you can't get anything into the brain. We're hoping to be able to deliver additional copies of glucocerebrosidase in order to at least cure the mouse model.
We have a mouse model of this where the mice only lived for 14 days after birth. We're using a vector called AAV9 which is Adeno-Associated Virus 9. People found a couple of years ago that when you injected this in the circulation, you could cross the blood-brain barrier and could actually deliver the genes to the central and peripheral nervous system. This is the main problem with neuronopathic Gaucher disease. you can't get anything into the brain. We're hoping to be able to deliver additional copies of glucocerebrosidase in order to at least cure the mouse model.
Chris - And the fact that you're going into a brain which is smaller than it will be when you're trying to cure a much bigger child or adult makes it easier because you're able to hit cells when they're at the stem cell stage, so then all of the daughter cells will inherit that change.
Simon - Well the thing is with the brain is that at birth, you're not actually adding any more cells or not adding many more cells to your brain. Your neurons are already in place so we are using a vector that doesn't integrate itself into the host genome, but actually because your brain is no longer dividing, these vectors can then stay around for the rest of the lifetime.
Chris - So you should get lifelong protection?
Simon - That's right, yes.
Chris - Any risks?
Simon - Well, I think as a community, we've always been very aware of any potential risks. So, the risks in terms of foetal gene transfer would be that you are also putting a needle into the mum and there's always a small, but still actual risk of damaging the foetus or causing birth.
Chris - Are you physically injecting this into the bloodstream of the foetus? Is that what you've got to do to get it into the brain? How are you doing that?
Simon - That's right. One of the big problems with brain gene therapy has been that you have to stick a needle into the brain in multiple sites because it doesn't spread through the brain naturally. Whereas these new and exciting vectors, you can put it into the bloodstream and then it can actually deliver genes throughout the entire brain and to the spinal cord.
There's some exciting work coming out on spinal muscular atrophy in mouse models and actually in larger animal models. In the mouse model, actually they cured the disease - the spinal muscular atrophy - which was completely stunning. And so, these researchers are actually now looking at the large models before considering clinical trials.
30:36 - Gene Therapy for Inherited Blindness
Gene Therapy for Inherited Blindness
with Robert MacLaren, University of Oxford
Rob - What we've been doing this week is very exciting. We've actually used gene therapy to attempt to correct an inherited eye disease, a type of retinitis pigmentosa. We've done this on a 63-year-old gentleman - Jonathan Wyatt - who suffers from a condition known as choroideraemia. Choroideraemia is one of those inherited diseases that affects men because it's on the X chromosome. It presents in childhood and initially starts with people losing their night vision and then gradually, as they get into their teens, they notice that the peripheral vision disappears and they eventually develop tunnel vision. So they can just see a tiny island of vision in the centre and then around about the age of between 40 and 50, that very small island of vision then disappears. Mr. Wyatt only has a tiny bit of vision left in the very centre of his eyesight and the operation we have done is using gene therapy. We have tried to replace the gene that's missing in him in order to preserve that little island of vision and keep it alive.
Chris - Why does this condition happen in the first place? Do we understand  what the disease process is that makes that progressive loss of vision happen?
what the disease process is that makes that progressive loss of vision happen?
Rob - Yes absolutely, effectively the protein that is missing is involved in moving vesicles around cells. Vesicles are like little compartments that move around carrying proteins from one part of the cell to another. The actual protein missing in choroideraemia is a protein that somehow labels the vesicle so that they know where to go so that they could be transported correctly.
Chris - And why does that lead to the pattern of visual loss that the patients have?
Rob - That is something which we're not entirely sure of, but it seems to be that this particular protein is absolutely critical in the retina in the eye. There is another protein which can take on the role of labelling these vesicles in other cells in the body, but in the eye, this other protein doesn't work as well and so, the patients do have the effect. It's interesting because it is a disease, like many genetic diseases, which is only manifested in the eye, and doesn't really have any effect elsewhere in the body.
Chris - So how have you tried to tackle it?
Rob - What we've done is we've engineered an adeno-associated viral vector which is a very small viral particle and inside that, we've packaged the missing gene. We like this particular viral vector because we do have good safety data on it and we know that it can be injected into the retina without causing any significant side effects. We're using the vector very much like a Trojan horse to get through the cell's defences and then open up inside and release the gene that's missing in choroideraemia into the cells. Hopefully, that will be sufficient to allow the cells then to continue functioning normally, and keep them alive.
Chris - Which cells is it going into?
Rob - Well we're targeting the lining of the retina, which is known as the retinal pigment epithelium. But also, we have made a fairly significant step forward in gene therapy in the eye, in that we have modified the vector slightly so that it infects the light sensitive cells, the photoreceptors which line the eye, and in order to do that we haven't changed the shape of the virus, we have actually changed the DNA sequence a bit so that the virus and the gene is expressed within the photoreceptors, particularly the rods which are those responsible for night vision in addition to the lining of the eye.
Chris - Does this mean that the progression of the disease is arrested - that the vision will stop getting any worse - or does it mean that actually, the vision can recover in affected people?
Rob - Well again, that is a very good question and probably we will only know for sure in the midst of time when we've had the chance to see the results. But what we predict to happen is we think that the disease will be arrested because it's a relatively slow degeneration and we think that even a small amount of the protein expressed would keep the cells alive. But what we've noticed is, because children, boys, lose their night vision before there's any significant degeneration of the retina, we think that there is some functional defect. In other words, that the actual rod photoreceptors themselves do not work as well in the absence of this protein. We've designed our study so that we can do quite complicated visual tests in the aftermath of the gene therapy to see if this night vision returns. And of course, what's really nice about working in the eye is that we've treated one eye in all our patients, we can then compare the treated eye to the other eye which hasn't received the vector. So we'll be sure whether or not the effect has worked.
Chris - And practically speaking, how do you actually get the virus into the eye?
Rob - Well the virus goes in through quite a complicated procedure where we make three small incisions in the white of the eye which is just behind the coloured part - the iris - and we pass these probes into the eye. We remove the jelly from the back of the eye first of all and then we inject fluid underneath the retina through a very, ve ry small needle which is actually thinner than a human hair. [This fluid ] lifts the retina up and it detaches it. It would be a bit like operating inside a tyre where we're actually trying to remove the inner tube and we want to inject the viral suspension between the inner tube and the outer wall of the tyre. So we have to make sure that we don't make too big a hole in the inner tube and also, we need to separate the inner tube from the outer wall of the tyre. One of the difficult things which we haven't been able to predict is how these patients with choroideraemia, whether or not these two layers of the retina will be stuck together because of course, with all the inflammation in the cells that's been going on throughout their lives, it's possible that the retina will be quite heavily struck together. And this is a bit unpredictable, but fortunately, in the case of Mr. Wyatt, the first patient we treated this week, the retina separated very cleanly and we were able to inject the virus into the correct compartment without any complications.
ry small needle which is actually thinner than a human hair. [This fluid ] lifts the retina up and it detaches it. It would be a bit like operating inside a tyre where we're actually trying to remove the inner tube and we want to inject the viral suspension between the inner tube and the outer wall of the tyre. So we have to make sure that we don't make too big a hole in the inner tube and also, we need to separate the inner tube from the outer wall of the tyre. One of the difficult things which we haven't been able to predict is how these patients with choroideraemia, whether or not these two layers of the retina will be stuck together because of course, with all the inflammation in the cells that's been going on throughout their lives, it's possible that the retina will be quite heavily struck together. And this is a bit unpredictable, but fortunately, in the case of Mr. Wyatt, the first patient we treated this week, the retina separated very cleanly and we were able to inject the virus into the correct compartment without any complications.
Chris - So when you're doing all this, are you physically watching what's happening by looking through the pupil at the back of the eye so you can see where these needles are going and what the effect is of the injections?
Rob - Yes. We have a very high-powered microscope which has a series of lenses on it. Most importantly is that it inverts the image, so when we look into the eye, we are seeing the image the correct way around. Otherwise, we'd have to do everything back to front which would be quite difficult. In the operation itself, what I have in my left hand is a tube which like a torch, a very high-powered torch, which I use to light up the back of the eye. In my right hand I have the actual needle with the very small tip which goes under the retina. By looking down the microscope, I can actually see myself moving these two instruments around. My feet are controlling various other parts of the microscope and the injection system which allows me to deliver the fluid into the retina without having to fumble around with another hand.
Chris - So not too much coffee in the morning before you do that. What are the outcome measures? How will you follow up this initial patient and then the others in order to test whether or not it's working and when will you know?
Rob - Well I think the main thing that we're interested in is first, that the patient comes to no harm and that his vision returns, and that the operation itself does not cause damage, and the viruses are delivered without any problems. I can say fairly confidently now that that is the case indeed and he was interviewed live on the BBC News on Thursday evening and gave a very good account of having good vision in the eye, and that's very reassuring. The second stage will then be to look at his night vision and also to have a look at his retinal function. We do that with a machine called a microperimeter which is a machine which shines a light in different parts of the vision, and the patient will then press a button when he or she sees the light. The light that's shone in the eye is also tracked with an infrared camera, so a computer knows exactly where on the retina the light is being shone and can correlate that with the patient's responses. In the long run, we know that this condition has a progression of about 10% per year in terms of reduction and shrinkage in the size of the retina and we can measure that on the back of the eye. So by measuring the retina that we treat, we have a very objective test about whether or not the disease progression has been halted because of course, we can compare the treated retina to the untreated eye on the other side, where we would expect that 10% per year shrinkage to continue.

38:45 - Fossil Proteins - Planet Earth Online
Fossil Proteins - Planet Earth Online
with Roy Wogelius, University of Manchester
Roy - What we're looking at now is a very, very small, probably juvenile reptile from the green river formation, that's about 50 million years old and about 5 centimetres long. It's only part of an organism and we think this poor unfortunate little critter probably got bitten in half and that's why it's ended up in the fossil record.
Richard - If we look at it, there's almost a long tail and two legs, I guess, almost like frog's legs but probably about half the length of my finger imbedded in this quite thin, sandy, almost like a slate [material].
I guess, almost like frog's legs but probably about half the length of my finger imbedded in this quite thin, sandy, almost like a slate [material].
Roy - That's right. You can see a long central tail and then you can see where the legs join in, where the pelvic region is and then the organism is truncated. Mostly what you can see is the skin. And if you look at it very, very closely you can actually see that there is some of the patterning left in the skin.
Richard - It is mottled isn't it?
Roy - Yes that's right, and that's the scale pattern. And that's why we thought this would be a tremendous specimen to look for the residue and patterning of proteins.
Richard - You're actually looking for remnants of proteins of the molecules that made up the skin?
Roy - Some remnants of the original chemistry.
Richard - After 50 million years.
Roy - That's exactly right. It's the chemical fossil. Now that seems like an outrageous thing to propose doesn't it? Yet we have absolutely no problem thinking about organic molecules being preserved as long as we don't think about what species they've come from or from exactly where they're from. We just put them in our gas tanks or petrol tank and burn them. This is a chance, using some of these very, very sophisticated techniques to track back and find some of these very, very robust organic molecules and trace them back to the their source and indeed, we were able to do that.
Richard - So what did you do? It's a very different process to the sorts of things palaeontologists normally get up to.
Roy - And that's why I describe myself as a geochemist. Usually, palaeontologists look at bone and then they look at structures. What we wanted to get to was using chemical techniques and we had this idea of using a different part of the electromagnetic spectrum. It's a very, very simple thing that we did: we just used infrared light rather than visible light. Now, infrared light gives you an idea about the presence of organic molecules because an awful lot of the infrared spectrum will cause vibrations in the organic components. And by that, what that means is that we can identify specific parts of organic molecules and the specific parts of the organic molecules that are present within this fossilised lizard skin are extremely similar to the organic molecular fragments that are present in beta carotene from existing lizard skin.
And so, we did a comparison of the distribution and types of these organic - we'll call them functional groups. We mapped these organic functional groups and compared them from this fossilized skin to skin taken from a present day gecko and the distribution patterns map very, very nicely.
Richard - So what can you conclude then?
Roy - What that showed us is that the protein residue derived from the original skin still has some of the character of the original proteins and the distribution of it is controlled by the original biological structure. It can and it will have a big, big impact on understanding evolution because we can get down to these protein sequence levels. Preservation of DNA, that's just not going to back into deep geological time, but the preservation of some of these proteins from these soft tissues does.
Richard - And this has applications beyond just understanding evolution.
Roy - One of the things I'm very interested in, in fact the other side of my research has to do with radioactive waste disposal and how we can safely sequester radioactive waste. Now, the safety cases, for most countries, have to demonstrate containment for between 100,000 and a million years. Well this is a 50-million year experiment between trace metal contaminants and organic compounds that tells us one way that nature has been able to sequester organic compounds and trace metals in place and that's very, very useful information for us.
44:01 - Treating Respiratory Disorders with Gene Therapy
Treating Respiratory Disorders with Gene Therapy
with Maria Limberis, University of Pennsylvania
Maria - My interest has been in cystic fibrosis (B) but more recently I have started to work in gene therapy for eye diseases.
Chris - Can you just review for us what we understand about cystic fibrosis - what it is, what causes it, why it happens.
Maria - So cystic fibrosis is a very devastating disease. It affects Caucasian populations and the frequency is really high for Caucasian populations, so 1 in 2,000 live births is affected with CF. There's no cure for the disease and for someone to imagine the impact of the devastation of lung disease, you just think to your worst cold ever, your worst chest cold ever, and that progressively getting worse. Patients basically don't have treatment and when lung disease becomes so bad, the only option is a lung transplant and of course, lung transplants...
for the disease and for someone to imagine the impact of the devastation of lung disease, you just think to your worst cold ever, your worst chest cold ever, and that progressively getting worse. Patients basically don't have treatment and when lung disease becomes so bad, the only option is a lung transplant and of course, lung transplants...
Chris - There are not many of those going around, are there?
Maria - Exactly, lung transplantations are down. So it's a very poor outcome for patients with severe lung disease.
Chris - At a molecular level, what do we understand about what's causing the condition?
Maria - So the gene was identified almost 20 years ago, 22 years ago to be exact, and it's a gene that encodes a chloride channel. The chloride channel has got a very fancy name - the cystic fibrosis transmembrane conductance regulator (CFTR)and it's situated on the top of the epithelial cells which are cells that line the lungs. And the function of this channel is to control the fluid that lines our lungs which allows us not to get colds when we walk into environments that are either dusty or full of bacteria and viruses.
So, the first thing that you do when you walk into a dusty room is cough and that's the natural defence of your lung to get rid of what it's inhaled. In the case of cystic fibrosis where those patients don't have that gene and the product, the lungs don't have the normal function of actually trying to get rid of all those noxious particles that they inhale. Which means that everything that they inhale remains in the lungs and whether it's bacteria or viruses, they replicate, they make a really nice home for themselves and progressively worsen the lung disease.
Chris - I'm quite glad you brought up the idea of how lungs defend themselves normally because that must be a big challenge for a gene therapist to need to overcome. Lungs are pretty good at preventing themselves from getting infected because they have all these defences - mucous and they have this mucous ciliary escalator that washes the mucous out - so anything you want to try and get into a lung cell has got to bypass all those defences first.
Maria - Exactly, exactly right. So, the same way that our lungs protect us from those daily encounters with the bacteria and the viruses, they don't have the ability to know the difference of a gene therapy vector versus something noxious that they've inhaled. So the same defences that apply to those noxious particles will apply to a gene therapy vector. So what we need to do when we develop the gene therapy vectors, and this is work that we've constantly been doing in the field is develop vectors that actually can bypass those barriers, and if we can't find vectors that can bypass those barriers, we try to momentarily disrupt those barriers.
Again, those barriers are there for a reason and that's to protect us from noxious particles, we don't want to introduce them along with the gene therapy vectors in those patients that will be susceptible to infections.
Chris - Some people are trying to persuade the lung cells to take up individual bits of DNA. Others are using viruses to do this. So is that your approach?
Maria - Yes. So we're using viruses. We believe that vectors that are based on viruses are very effective. We've all had our common cold and we've had it more than once and a common cold is very effective, you see the symptoms straight away, and it spreads throughout the lung very quickly. So we're trying to harness that ability of a virus to get into the cells of the epithelium, the cells of the lung to actually use that.
Chris - Which viruses are you using?
Maria - So we're using a small virus. It's called an adeno-associated virus. It's never been associated with any human disease. It's effectively been used and translated into clinical trials and in fact, there have been recent successes with eye diseases using this particular virus.
Chris - So how are you using it - what do you need to do to that virus so that you can get genes into the lung cells that need them?
 Maria - So in terms of the virus, what you do is you sort of remove what you consider the virus to have in terms of pathogenic genes. So you remove those genes that are pathogenic that could potentially cause disease and you replace them with a therapeutic gene - in this case, cystic fibrosis gene - but you allow those genes that are basically necessary for that virus to attach onto the cell, to move through the cell, to go and integrate, or stay close to the DNA of the host cell.
Maria - So in terms of the virus, what you do is you sort of remove what you consider the virus to have in terms of pathogenic genes. So you remove those genes that are pathogenic that could potentially cause disease and you replace them with a therapeutic gene - in this case, cystic fibrosis gene - but you allow those genes that are basically necessary for that virus to attach onto the cell, to move through the cell, to go and integrate, or stay close to the DNA of the host cell.
So, we try to harness what's good about the virus and remove what's bad about the virus - remove the bad part of the virus and incorporate the therapeutic gene. And so, in essence, the same way that you would get your common cold and all those symptoms, you will now replace the common cold with the cystic fibrosis gene.
Chris - So the virus that came in from the cold if you will. The problem I can see with this though is that the cells that you're going to hit with your therapy because this is something a patient is going to breathe in or have washed into their lungs isn't it - you're going to hit cells that are short-lived.
Maria - Correct.
Chris - And that means that you're going to have to keep repeating this therapy. So I can see sort of two problems - one, that's an inconvenience and two, won't the patients become immune to continual exposure to this virus and they'll just develop antibodies and that will prevent you doing this?
Maria - Correct. So, depending on the virus that you use, it has the ability to stably integrate into the genome, into the DNA of that cell. Now in the case of - for example, a ciliated cell which is the cell that actually lies on the surface of your lung - that's considered a terminally differentiated cell which means that once that cell dies, it does not give rise to any gene-corrected cells. We still don't know, even though there are hints at what is the actual stem cell, the progenitor of the airway, but with the viruses that we have in hand, we don't only target one specific cell type. That's the beauty of the viruses that we use, that we can target several different types of the airway epithelium. Now ideally, you'd like to target the stem cells.
We don't know which ones those are, but we're hoping that with the strategies that we're using, at least one population or subpopulation will have the ability to become a stem cell or to give rise to gene corrected cells. Now in the event that you don't transduce or target those stem cells, you will need to re-administer your virus. Now, we do have some window, some time in terms of getting a re-administration. These cells live between 3 and 6 months, so with certain viruses, the neutralising antibody comes down, to a low level, that will allow re-administration of the virus.
Chris - You can get around it just because the immune system loses interest.
Maria - Exactly!
Chris - And you can come back in and hit it again.
Maria - Exactly!
Chris - Wouldn't a better way though when you sort of hinted at this with the stem cell idea - wouldn't a better way be to inject the virus into the bloodstream and program it to go for the stem cells in the lung, assuming we can find them? So that you would hit the stem cell, and then it would propagate into all of the daughter cells the beneficial change that you've impregnated it with effectively?
Maria - Yes, that could be an option. The problem is that once you inject things into the systemic circulation if you will, everything gets cleared by the liver. And so, you'll either end up losing a lot of your virus that's swept by the liver, or you'll have to inject bucket loads of vector. Now the more vector you inject into a human, the more likely it is that you're going to have a devastating immune response just by the mere fact that you're injecting a lot more vector that you need. So while that could potentially be an approach, a more effective way is to actually try to localise your delivery to where you want the vector to actually transfer the gene of interest.
Chris - And to finish off Maria, how close are you to being able to do this in a human.
Maria - In terms of the work that we do in our lab, we focus on adeno associated viruses. We have collaborations with others that work on Lenti viruses. The field has made tremendous work and advances over the last 10 years. We're not ready for a clinical trial. We're still answering questions that have become apparent as a result of clinical trials that have not produced the results that we expected, and what that allowed us to do, if you will - the failures - even though that's potentially a wrong word for a clinical trial, the failures that became apparent allowed us to ask questions that have become very important as we try to move forward with gene therapy, which is trying to make our vector more efficient, trying to make a vector more safe.
And the truth of the matter is that you don't really know how well your vector is going to be unless you inject it in a human, and that's the case that's happened with many clinical trials, and the experiment actually starts when you inject to human. In terms of the progress in the field so far, there is an active gene therapy trial and that's the one from the UK Cystic Fibrosis Gene Therapy Consortium in which they're using a lipid-based approach to actually deliver plasmid and coding for the cystic fibrosis gene in patients with cystic fibrosis here in the UK.
Is it possible to alter genes on an orgamism-wide scale?
Maria - Potentially, if you were to go about some work that Simon's been doing in terms of going in prenatally. So we know for example that if you go prenatally with certain animal models and you inject a virus in systemic circulation, it will go everywhere so we can get transduction, positive expression of a particular gene in liver and the brain, and the lung - every single organ. But again, you have side effects associated with that depending on the gene and where it's expressed. You don't want to be expressing a gene in a location where it wasn't supposed to be expressed in the first place. But the technology is available to do something like that based on animal data.
How can we prevent the undesirable effects of gene therapy?
We posed this question to Simon Waddington and Adrian Thrasher from University College London...
Simon - Gene therapy and stem cell therapy researchers have spent a huge amount of time not only trying to make the most efficient gene therapy vectors in stem cell treatments but also to ensure that they are as safe as possible. And when we're using different gene therapy vectors - for example, if we're using ones that are based on HIV - these actually insert themselves into the genome and they can therefore disrupt the genome. So there's certainly been work over the past 8 years to try to firstly make them as safe as possible so they don't affect the surrounding genes. But also, as Kathy High alluded to in her presentation today, what we've been doing for many years in gene therapies actually is providing a supplementary copy of the gene, but actually, still leaving the mutated gene in. Whereas what Kathy is doing, and various other researchers in the community, is creating tool kits to be able to actually repair the gene and return it to what we would perceive to be its normal function. And that, although it's inefficient at the moment is very exciting as almost an ideal safe gene therapy.
Ben - And as with all the types of medicine, there must be all sorts of regulation, all sorts of boxes that you need to tick before you can actually put something on the market, or even before you can get it into trials in people.
Adrian Thrasher - Of course, gene therapy is no different from any other pharmaceutical therapy in terms of its regulation. We obviously have to go through a fairly rigorous ethical process and also be able to ensure that we can give medicines that are made or manufactured in the right way that's safe for people.
What about the epigenetic effects of gene therapy?
We posed this question to Professor Adrian Thrasher from Great Ormand Street Hospital and University College London...
Adrian - Epigenetics is increasingly important in our understanding of how genes work. It regulates in some ways how the genes are turned on or off, it's important through developmental processes. It's important in adult cells, in foetal cells. It's also important in gene therapy because we know if we don't get the sequences right, the genes can become epigenetically modified. - so may switch off unexpectedly which is undesirable. It may switch on unexpectedly which is also undesirable. So the epigenetics is important. There's a lot of ongoing work.
Can Gene/Stem cell therapy treat immune disorders?
We posed this question to Professor Adrian Thrasher from Great Ormand Street Hospital and University College London...
Adrian - Yes, that's certainly the case. Interestingly, the primary immune deficiencies have been one of the key disorders that has taught us proof of principle that we can successfully treat patients by gene therapy, 40 years back now by bone marrow transplantation. As we uncover the genetic mistakes that underlie these disorders, of course, we can apply the technologies to treat them. So common variable immunodeficiency is one of those disorders although we only understand genetic cause for about 10% of them. So over the next few years we will be able to apply genetic technologies, I'm sure.
![Cat Blue-eyed cats with white fur have a higher incidence of [[:en:genetics##genetic]] [[:en:deafness]]. Over 200 heritable genetic defects have been identified in the cat, many of which are homologous to human inborn errors. Specific metabolic defects...](https://www.thenakedscientists.com/sites/default/files/styles/medium/public/media/WhiteCat.jpg?itok=Yuk5YLst)
61:16 - Is human facial hair like a cat's whiskers?
Is human facial hair like a cat's whiskers?
We put this question to Nick Crompton, a Zoologist in the Mammal Evolution and Morphology group in Cambridge University... Nick - To a certain point, the whiskers on your cat's face and stubble on mine look pretty similar. After all, they're both part of our Pelage - our insulating furry coat. But whiskers are a type of specialised hair called vibrissae. Now among other places, they're found in the maxillary - that's the upper lip region, and they come in both macro and micro forms, and in the same way as normal hairs, they're keratinous structures growing out of the skin - the epidermis. But whiskers tend to be far thicker and as we know, they act as mechanoreception organs.
Now obviously, when someone pulls a hair out of my head it hurts, and to some extent, all hairs act as mechanoreceptors for very light touch. So, I can feel if something brushes very lightly up against the hairs on my arm. But vibrissae sport a suite of tactile sensitive organs at their base, so any tiny displacement results in a far more complicated signal being transduced to the animal's brain wherein a large portion of its somatosensory cortex is concerned with interpreting signals as spatial information.
Beardy chaps don't have this sort of specialisation within our brains and in fact, humans are almost unique in being one of only two mammals known not to sport any vibrissae at all, along with the anteater. But it's recently been reported that the muscles used to move whiskers around are present in the human lip, but has very degenerate vestigial structures. Now our beards are more than likely the result of sexual selection like the mutton chops and moustaches on some old world monkeys, and they just help their owners look dashing, if maybe, a little bit scruffy.
- Previous Do humans have whiskers?
- Next Cancer and Ocular Gene Therapies










Comments
Add a comment