Ingredients
 | A way of carbonating water - a soda syphon or sodastream |  | Some red cabbage |
 | Some glasses |
Instructions
Grate a small amount (2-3 leaves) of raw red cabbage and put it into a glass. Add some tap water and then mash it up as well as you can.
Strain out the lumps of cabbage to produce a clear solution. This is your pH indicator, it will behave like litmus changing colour depending on the acidity or alkalinity of your solution (to find out more see
this experiment).
Test some of your tap water by pouring a small amount of indicator (3-4mm) into a glass and adding tap water. If the solution is bluey purple then you have hard slightly alkaline water, if it is pink then you have slightly acidic water. We want the water to start off slightly alkaline, so add a little bicarbonate of soda to your tap water until it stops testing pink.
Split your water into two, carbonate half of it, and leave the other half alone.
Now test both the carbonated and uncarbonated water with your red cabbage indicator and see if there is a difference.
Result
You should find that the carbonated water makes the water much more acidic than the straight tap water.
This is the reason why if you taste the carbonated water it has a tangy, sharp - acidic taste.
First a bottle of normal water is added to the indicator, and then some carbonated water from the soda syphon
Explanation
When you carbonate water you are essentially passing high pressure carbon-dioxide through it and a large amount of that carbon dioxide dissolves in the water.
 | 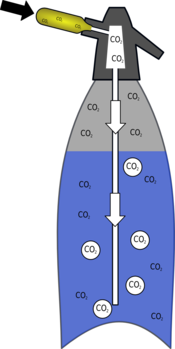 |
| The bulb in a soda syphon is basically just a high pressure carbon dioxide tank. | When you screw the bulb into the machine you break the seal and carbon dioxide is released. As it bubbles through the water most of it dissolves. |
Carbon dioxide is far more soluble in water than are similar gases such as oxygen or nitrogen. It can also react with the water to form dihydrogen carbonate (carbonic acid).
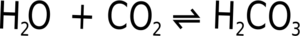
This reaction is reversible, it is continuously occurring in both directions, carbonic acid is being made and destroyed all the time. This means that if you increase the amount of carbon dioxide by increasing the pressure you will increase the speed of the production of carbonic acid reducing the amount of gas.
Similarly if you reduce the pressure you will slow the creation of carbonic acid, but it will keep on splitting up to form carbon dioxide gas. This is why if you rapidly reduce the pressure on a carbonated drink it can rapidly turn into foam (see the
lemonade volcano experiment).
Why is the carbonated water acidic?
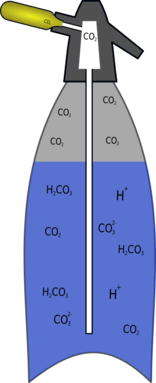 |
| The CO2 dissolves and reacts with water to form H2CO3 which disassociates to form carbonic acid. |
The hydrogen carbonate can break up (disassociate) in another way, it can split up into a hydrogen ion (H+) and a hydrogen carbonate (bicarbonate) ion (HCO3-).
![]()
Any solution containing a lot of free hydrogen ions is acidic. In fact pH (the acid-alkali scale) is just an obscure+ measure of the concentration of hydrogen ions in a solution.
The reaction is again reversible so if the amount of hydrogen carbonate reduces so does the amount of acid.
There is naturally carbon dioxide in the air, and this will dissolve in water making it slightly acidic. This is how water can dissolve away limestone to create caves. As we pump more carbon dioxide into the atmosphere it will make water more acidic, this may cause problems for shellfish whose shells are effectively made of limestone.
Are there actually free protons floating around in an acidic solution?
Strictly an H+ ion is a hydrogen atom missing an electron - a proton, which does sound unlikely. In fact what happens is that the Hydrogen carbonate reacts with water to form a hydronium ion (H3O+).
But all the maths works out the same if you think of hydrogen ions so mostly chemists do.
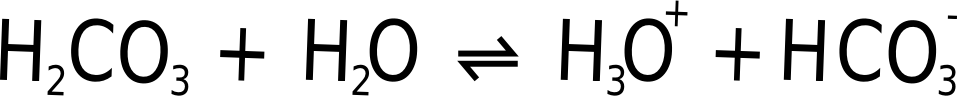
+ It is actually pH = - log10( [concentration of hydrogen ions / mol dm-3] ) ... as I may have said, fairly obscure. It so happens that it makes convenient scale and makes other maths easier for chemists.
- Previous Weighing the Atmosphere
- Next Why do wheels work?










Comments
Add a comment